NAD+ METABOLISM AND OXIDATIVE STRESS: THE GOLDEN NUCLEOTIDE ON A CROWN OF THORNS
Hassina Massudi1, 2, Ross Grant1, et al; 1Department of Pharmacology, School of Medical Sciences, University of New South Wales, Faculty of Medicine, Sydney, Australia, 2Australasian Research Institute, Sydney Adventist Hospital, Sydney, Australia, 3St Vincent’s Centre for Applied Medical Research, Sydney, Australia, 4School of Psychiatry, University of New South Wales, Faculty of Medicine, Sydney, Australia

NAD moleccule
In the twentieth century, NAD+ research generated multiple discoveries. Identification of the important role of NAD+ as a cofactor in cellular respiration and energy production was followed by discoveries of numerous NAD+ biosynthesis pathways. In recent years, NAD+ has been shown to play a unique role in DNA repair and protein deacetylation. As discussed in this review, there are close interactions between oxidative stress and immune activation, energy metabolism, and cell viability in neurodegenerative disorders and ageing. Profound interactions with regard to oxidative stress and NAD+ have been highlighted in the present work. This review emphasizes the pivotal role of NAD+ in the regulation of DNA repair, stress resistance, and cell death, suggesting that NAD+ synthesis through the kynurenine pathway and/or salvage pathway is an attractive target for therapeutic intervention in age-associated degenerative disorders. NAD+ precursors have been shown to slow down ageing and extend lifespan in yeasts and protect severed axons from degeneration in animal models neurodegenerative diseases.
Introduction
Current thinking regarding the importance of NAD (including NAD+ and NADH) metabolism in health and disease stems from the original discovery that niacins were efficacious for the treatment of pellagra.1 In 1937, Elvehjem2 showed that nicotinic acid (NA) and nicotinamide (NM) (the breakdown product of NAD+) were effective agents for both the treatment and prevention of black tongue in dogs, and the human equivalent, pellagra. While the disappearance of pellagra in the developed world reduced interest in this life-threatening deficiency disease, a recent observation that several disorders mimic pellagra in its range of symptoms (e.g. dermatitis, diarrhoea, dementia, and death) has re-ignited considerable interest on the mechanism of action of NAD+ and its key metabolites.3–7
Intriguingly, with the understanding of the pharmacological role of niacins was the almost simultaneous discovery of the structure and function of NAD+ by four Nobel laureates.8 In 1904, Sir Arthur Harden separated Buchner’s yeast juice into a high- and low-mol- ecular-weight fraction, neither of which could undergo fermentation individually. However, recombination of both fractions allowed fermentation to take place. Harden inferred the existence of high-molecular- weight ferment (enzyme) and a low-molecular-weight coferment or ‘cozymase’.8
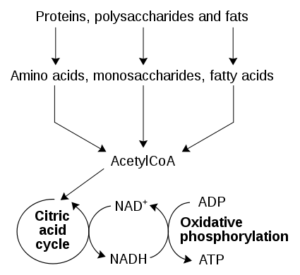 The involvement of cozymase in fermentation, respiration, and glycolysis was identified in a variety of organisms in subsequent years. However, due to the low-molecular-weight nature of cozymase, it was difficult to isolate.8 It was Hans von Euler-Chelpin9 who eventually succeeded in isolating cozymase from yeast extracts in the late 1920s. He determined the dinucleotide structure of NAD+ and two other mono-nucleotides, adenosine monophosphate (AMP) and nicotinamide mononucleotide (NMN).9 The central redox function of NAD+ was determined by Otto Warburg and Christian in the mid-1930s.10 He identified the capability of NAD+ to transfer hydrogen from one molecule to another.10 Since then, ongoing investigations have been directed towards the identification of the enzymatic pathways involved in its syn- thesis and metabolism.11 In 1954, Arthur Kornberg12 discovered the NAD phosphorylase reaction, the crucial step of NAD+ synthesis. He detected the enzymatic activity in yeast extracts which catalysed the condensation of ATP with NMN to form NAD.12 While this reaction is catalysed by nicotinamide mononucleotide adenylyltransferase (NMNAT) [EC 2.7.7.1], it took another 55 years for the primary structure of this enzyme to be discovered.13
The involvement of cozymase in fermentation, respiration, and glycolysis was identified in a variety of organisms in subsequent years. However, due to the low-molecular-weight nature of cozymase, it was difficult to isolate.8 It was Hans von Euler-Chelpin9 who eventually succeeded in isolating cozymase from yeast extracts in the late 1920s. He determined the dinucleotide structure of NAD+ and two other mono-nucleotides, adenosine monophosphate (AMP) and nicotinamide mononucleotide (NMN).9 The central redox function of NAD+ was determined by Otto Warburg and Christian in the mid-1930s.10 He identified the capability of NAD+ to transfer hydrogen from one molecule to another.10 Since then, ongoing investigations have been directed towards the identification of the enzymatic pathways involved in its syn- thesis and metabolism.11 In 1954, Arthur Kornberg12 discovered the NAD phosphorylase reaction, the crucial step of NAD+ synthesis. He detected the enzymatic activity in yeast extracts which catalysed the condensation of ATP with NMN to form NAD.12 While this reaction is catalysed by nicotinamide mononucleotide adenylyltransferase (NMNAT) [EC 2.7.7.1], it took another 55 years for the primary structure of this enzyme to be discovered.13
Another important milestone was the discovery of the NAD+-consuming activity associated with the transfer of ADP-ribosyl moieties to protein acceptors which occurred in the 1960s.14 In the same period, the term ‘pyridine nucleotide cycle’ was introduced to define several enzymatic reactions involved in the biosynthesis and catabolism of NAD+, although the significance of NAD+ metabolism remained unclear.15 Rechsteiner and Catanzarite (1974)16 showed that NAD+ turnover was strongly suppressed in enucleated yeast cells, suggesting that the nucleus is the major compartment responsible for the anabolism and breakdown of NAD+. Since then, studies on the enzymology of mammalian NAD+ metabolism have increased in line with those investigating the potential involvement of NAD-related metabolites in cellular physiology.11,17,18 The involvement of NAD+ in several key cellular processes has suggested to some the possibility that NAD+ metabolism may be an attractive therapeutic target.19,20
Changes in NAD+ metabolism have been associated with several pathologies, including neurodegenerative diseases, cancer, cardiovascular disease, and normal ageing. A number of comprehensive papers have appeared in the literature, which provide a generalized overview of the information in this field.11,17,18,21 Beginning with an overview of relevant background research by others, this review provides additional insight into the involvement of NAD+ metabolism in neurodegenerative disorders and ageing.
READ MORE NAD+ metabolism & oxidative stress

