THE HORMONAL PATHWAY TO COGNITIVE IMPAIRMENT IN OLDER MEN
MAGGIO, et al
Department of Internal Medicine and Biomedical Sciences, Section of Geriatrics, University of Parma, Parma, Italy; 2. Geriatric Unit and Laboratory of Movement Analysis, Geriatric-Rehabilitation, Department, University Hospital, Parma, Italy; 3. Department of Neuroscience, University of Parma, Parma, Italy and Clinical Neuroscience Centre, University of Hull, UK. Corresponding author: Marcello Maggio, MD, PhD Department of Internal Medicine and Biomedical Sciences, Section of Geriatrics, University of Parma, via
RONALD PETERS MD – COMMENTARY
Catabolism is the “breaking down” or “recycling” process that removes waste at the cellular level and generates energy for cellular activities. Anabolism is the process that creates molecules that “builds up” tissue thereby supporting growth and development. Cortisol and thyroid are catabolic, while DHEA, testosterone and insulin like growth factor (IGF-1) are anabolic. Proper balance for these two essential life functions is key to health throughout life. Integrative medical practice evaluates this balance in every patient in order promote health in body and mind.
Abstract: In older men there is a multiple hormonal dysregulation with a relative prevalence of catabolic hormones such as thyroid hormones and cortisol and a decline in anabolic hormones such as dehydroepiandrosterone sulphate, testosterone and insulin like growth factor 1 levels. Many studies suggest that this catabolic milieu is an important predictor of frailty and mortality in older persons. There is a close relationship between frailty and cognitive impairment with studies suggesting that development of frailty is consequence of cognitive impairment and others pointing out that physical frailty is a determinant of cognitive decline. Decline in cognitive function, typically memory, is a major symptom of dementia. The “preclinical phase” of cognitive impairment occurs many years before the onset of dementia. The identification of relevant modifiable factors, including the hormonal dysregulation, may lead to therapeutic strategies for preventing the cognitive dysfunction. There are several mechanisms by which anabolic hormones play a role in neuroprotection and neuromodulation. These hormones facilitate recovery after brain injury and attenuate the neuronal loss. In contrast, elevated thyroid hormones may increase oxidative stress and apoptosis, leading to neuronal damage or death. In this mini review we will address the relationship between low levels of anabolic hormones, changes in thyroid hormones and cognitive function in older men. Then, giving the contradictory data of the literature and the multi-factorial origin of dementia, we will introduce the hypothesis of multiple hormonal derangement as a better determinant of cognitive decline in older men.
Introduction
Decline in cognitive function, typically memory, is a major symptom of dementia. The “preclinical phase” of detectable cognitive impairment precedes by many years the appearance of dementia (1, 2). The decline of cognitive function with age affects different domains including selective attention, processing capacity and speed, verbal fluency, complex visual and spatial skills, and language analysis (3). There is evidence that a preclinical or sub-clinical phase of reduced cognitive function, such as information-processing speed (4-5), precedes the appearance of diagnosed dementia by at least 10 years. The decline in these domains begins as early as the mid-twenties and continues in dysregulation. In older men there is a multiple hormonal dysregulation with a relative prevalence of catabolic hormones such as thyroid hormones and cortisol and a decline in anabolic hormones such as dehydroepiandrosterone sulphate (DHEAS), testosterone (T) and insulin like growth factor 1 (IGF-1). We will underline the role of anabolic deficiency and namely of the decline in anabolic hormones such as testosterone, IGF-1, and DHEAS in older men. The profound interrelationship between these 3 hormones (DHEAS can be converted into testosterone and estradiol and both hormones affect the liver production of IGF-1) may explain the reason to include all these hormones in the present review (Figure 1). To complete the picture, giving the higher prevalence of subclinical dysthyroidism in older patients, we will also address the role of thyroid hormones on cognitive function.
Since literature provides already evidence of a relationship between chronic stress, elevated cortisol levels and risk of cognitive impairment and dementia (10-12), the link between cortisol and cognitive functions will not be examined here. We will focus on the decline in anabolic hormones introducing the new concept of multiple hormonal dysregulation as a potential factor involved in the onset of cognitive decline in the elderly.an almost linear fashion in the last decades of life (6). Many factors including health status, socioeconomic factors, lifestyle, and genetics contribute to inter-individual differences in the rate and severity of cognitive decline. Identification of relevant modifiable factors may lead to new strategies for preventing the age-related cognitive impairment. The Intervention on Frailty Working Group recognized that both physical and the cognitive problems play an important role in frailty and disability with a close relationship between frailty and dementia (7). The development of frailty may be due to a cognitive impairment (7), whereas recent findings suggest also that frailty could contribute to cognitive decline (8). As already shown in studies testing the role of the hormonal pathway in frailty (9), one of the potential factors both influencing normal and pathological cognitive ageing is the hormonal
DHEAS and cognitive function
Neurobiological actions
Dehydroepiandrosterone (DHEA) and its sulfate derivative DHEAS are the major secretory products of the adrenocortical gland, and are produced in larger quantities than any other steroid hormone. The levels of these two hormones decline with age in both sexes. The fall in circulating levels of DHEA/S is concomitant with the onset of many common physiological and functional impairments associated with age (13-15).
Figure 1
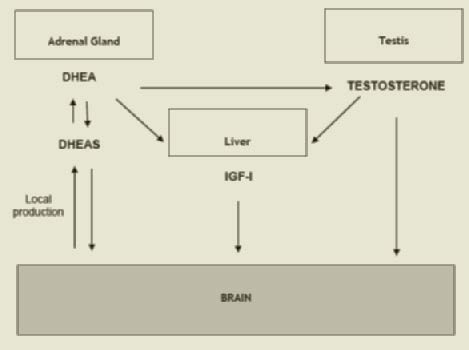
The putative positive effects of DHEA/S in the brain include neuroprotection, neurite growth, neurogenesis and neuronal survival, apoptosis, modulation of catecholamine synthesis and secretion, anti-oxidant, anti-inflammatory and antiglucocorticoid effects (16) .
The mechanisms by which DHEA/S operates in brain are not fully understood.
In animal models DHEA/S could operate its neurobiological functions through genomic and non-genomic mechanisms
Figure 2
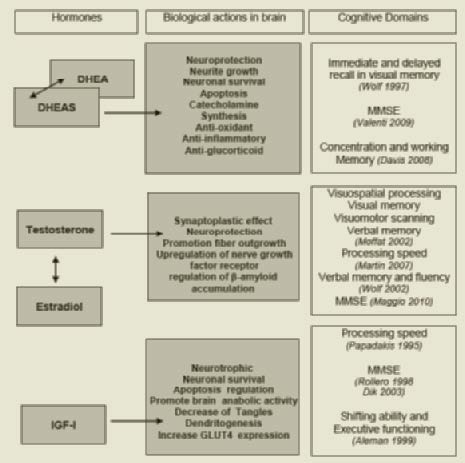
At genomic level DHEA/S may decrease neuronal death, enhance astrocitic differentiation (17), induce cortico-thalamic dendritic projection growth (18), and facilitate transcription of genes that lead to the synthesis of enzymes and structural proteins (19).
At non-genomic level, the actions of DHEA/S include allosteric antagonism of γ-Aminobutyric acid (GABA) receptor (20), activation of N-Methyl-D-aspartic acid (NMDA) receptors (21), interference with muscarinic acetylcholine receptors (22), dopamine and serotonine agonistic effects (23), attenuation of oxidative stress and neurotoxic effects of certain aminoacids (24-25), stimulation in the production of IGF-I, vascular endothelial growth factor (VEGF), transforming growth factor (TGF) beta 1 (26) and antagonism of glucocorticoid exposure (27).
DHEA/S may mediate some of its actions through conversion into sex steroids and activation of androgen and estrogen receptors at tissue level.
Morley et al showed that the rate of decline in bioavailable testosterone levels is paralleled by decline in its precursor DHEAS, suggesting that decreased synthesis of DHEA can contribute to the reduction in testosterone levels (28).
Moreover, in mice, estrogens may potentiate the effects of DHEA on brain. In this animal model DHEAS injection into the hippocampus improved memory retention in a dose dependent manner (29).
In addition, DHEA/S may also have effects through its more immediate metabolites, such as 7-hydroxy-DHEA. Although no DHEA or DHEAS nuclear steroids receptor has been found, DHEA and DHEAS show affinity for some binding sites.
DHEA and DHEAS have different effects on neural survival, suggesting that the balance between these two neurosteroids may play a critical role in nervous system development and maintenance (16).
Observational studies
Several human studies have investigated the relationship between the age-related DHEA/S decline and cognitive impairment. No significant association between DHEAS and cognition has been found in two large prospective cohort studies in men: 1) men and women of Rancho Bernardo Study (30); and 2) men in the Baltimore Longitudinal Study of Aging (31).
Moreover in the large older male cohort of Massachusetts Male Aging Study no significant correlation was found between DHEA/S and cognition (32).
These data were not confirmed in a second large population study of older women showing a positive relationship between DHEAS levels and some cognitive domains (simple concentration and working memory) (33) (Table 1).
A trend toward an inverse association between DHEA and rate of cognitive decline was detected in a French community based cohort study (34) and in a small sample of healthy older subjects of the population-based Rotterdam study (30).
Valenti et al recently reported the association between DHEAS and cognitive function over time, by analyzing data from the population-based sample of the InCHIANTI Study.

