COCONUT OIL AS A TREATMENT FOR ALZHEIMER’S DISEASE: A REVIEW
Poorni Sandupama 1, Dilusha Munasinghe 1, Madhura Jayasinghe Journal of Future Foods, Volume 2, Issue 1, March 2022
Dr. Peters’ Commentary
Dr Mary Newport reversed her husband’s Alzheimer’s disease with coconut oil which provided ketones as an alternative brain fuel, thus improving his memory. The ketogenic diet has been used to treat epilepsy since 1921 as well as children with autism spectrum disorder (ASD) since 2003. The ketogenic diet provides benefits in the short term, but is not recommended for long term use. However, organic coconut oil provides ketones as a nutritional supplement, which can also benefit people with Parkinson’s disease, amyotrophic lateral sclerosis and schizophrenia.
Abstract
The coconut tree (Cocos nucifera) which is also known as the “Tree of life” has its own values in each part of the tree and coconut oil is more prestigious among them. At present, the consumption of coconut oil is booming all around the world owing to its tremendous health benefits. The unique chemical composition of coconut oil enriched with medium chain fatty acids (MCFAs) has led to the exploration of these nutritional and therapeutic influences. Unlike the long chain fatty acids (LCFAs), the MCFAs generated from the digestion of medium chain triglycerides (MCTs) has a specific pathway for the metabolism, as it bypasses the lymphatic system and enter the liver directly through the portal vein. Due to such distinct attributes in absorption and metabolism, MCTs are readily capable of forming ketone bodies than other triglycerides. These ketone bodies are a competent energy source for the brains, especially those having cognitive impairments like Alzheimer’s disease (AD). AD is a neurodegenerative disease characterized clinically by accelerating shortfalls in memory and behavioral changes. The principal biochemical hallmarks behind the pathogenesis of AD are the development of extracellular amyloid β plaques and the accumulation of intracellular neurofibrillary tangles. Occurrence of Cardiovascular diseases (CVD) with elevated LDL levels, hypertension, Type 2 diabetes, obesity, and insulin resistance are some key risk factors that are responsible for the increasing prevalence and incidence of AD. There is sufficient evidence to prove that MCTs in coconut oil are metabolized and absorbed in such a way that retards the severity of these physiological risk factors. Besides, coconut oil is endowed with many polyphenolic compounds that are serving as antioxidants by combating oxidative stress and inflammation, which in turn downregulates the etiology of AD. But depending on the different processing conditions applied in extraction techniques of coconut oil, variations in antioxidant capacity can take place. Even though there are inadequacies in peer-reviewed large cohort clinical data for the long run, this article reviews that coconut oil, its constituents, and metabolism have positive findings on the potentiality to treat AD as a nutritional supplement.
-
Overview of Alzheimer’s disease
Alzheimer’s disease (AD) was first described by the German psychiatrist and neuropathologist, Alois Alzheimer, in 1906 [1] and is considered as one of the most commonly seen progressive neurodegenerative disorders in elderly populations [2].
AD can be diagnosed up to a sufficient level, with the proper investigation of symptoms developing in the suspected individuals. Early clinical symptoms involved in most of the patients are; depression, apathy, and struggle in remembering recent incidents with the gradual progression of the disease, confusion, poor communication, disorientation, behavioral changes, and eventually, troubles in walking, swallowing like bodily functions arise as later symptoms [3].
Moreover, AD is the largest unmet medical need in neurology which is clinically characterized by a progression from episodic memory problems to a slow global decline of cognitive function that leaves patients with end-stage AD bedridden and dependent on custodial care, along with death occurring on average 9 years after diagnosis [4].
The subjects who are suspected as AD patients can be detected mainly via the shrinkages in brain regions, especially responsible for the memory and learning, on magnetic resonance images and elevated absorption of radioligands that spot unusual amyloid depositions on positron emission tomography scan [5].
Intracellular neurofibrillary tangles [6], as well as extracellular accumulation of plaques composed of amyloid β (Aβ), are the main pathological hallmarks of AD [7]. Various risk factors like age, lifestyle and dietary patterns, environment, genetics, and different metabolic diseases can increase or decrease the development of AD in each individual [8].
The prevalence of AD is increasing at a phenomenal rate, affecting more than 40 million people all around the world [9]. Generally, the potential risk is desperately striking the elderly individuals beyond the age of 70 [10], and it was estimated that there is a prevalence of 10%‒30% in this aged population, while having an incidence of 1%‒3% [11]. There are two main types of AD, though the effects of the disease are similar [12]. Early-onset familial AD is one type that is characterized by dementia onset at a relatively young age (before 65 years of age) and positive family history for dementia. Familial AD, early-onset AD, and autosomal dominant AD are some terminologies that are used to describe these entities. If we use the stringent criteria of early-onset AD at age < 61 years, the prevalence is 41.2 per 100000 persons, respectively [13].
The next type is late-onset Alzheimer’s which is the most common form of the disease, happens to people age 65 and older. There is enough evidence that early-onset (sporadic) AD overlaps with normal aging in many clinical and pathologic aspects [14]. In general, early-onset AD accounts only for 5% of total AD cases. The majority of AD patients (90%–95%) are late-onset AD patients who usually develop AD after 65 years of age [15].
Doubling time is a fine indicator for estimating the future disease prevalence and the recent findings stated that, approximately every 5‒6 years, the AD incident rate doubles in magnitude [16]. According to the Alzheimer’s Association, the incidence of AD will escalate threefold in the coming 50 years and it can be considered as a piece of evidence that proves the severity of this disease [6]. If the enormous implications of this epidemic continue with society, it is predicted that by 2050, in every 33 seconds, one new incident of AD is anticipated to arise while subsequently expanding the number of annual cases around one million [17]. Therefore, the perilous risk of disease occurrence will enhance continuously, unless novel therapeutic inventions facilitate minimization or avoidance of the disease.
-
Pathogenesis of AD
AD is a gradually intensifying neurodegenerative disease [18], in which the major brain-related pathological hallmarks are the aggregation of the protein fragment Aβoutside the neurons and the formation of intracellular neurofibrillary tau tangles (NFT) [19].
Generation of Aβ proteins mainly depends on the expression of APP (amyloid precursor protein) on the cell surface [7] by enzymatic cleavage entailing β-secretase and γ-secretase activities [20].
Senile plaques are configured in the walls of cerebral blood vessels, as a result of the polymerization of these Aβ peptides into insoluble filaments [21]. The generation and aggregation of Aβ is the central incident that triggers, formation of tau tangles, apoptotic cell death [22], acute synaptic toxicity [4], inflammation, and neuronal atrophy during the final phase of the disease [23].
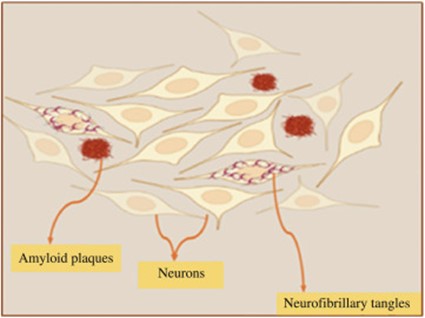
Fig. 1. Graphical representation of accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles (in the cerebral cortex of an AD brain).
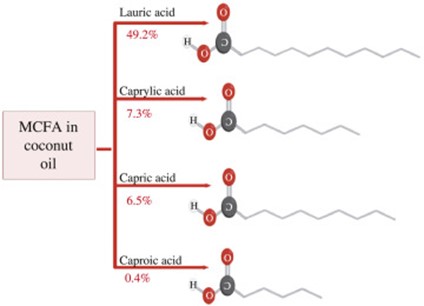
Fig. 2. Prominent types of MCFAs in coconut oil, their relative proportions, and molecular structures.
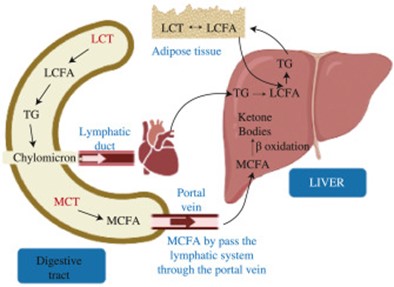
Fig. 3. Graphical illustration of the metabolism of MCT compared to the LCT within the human body.
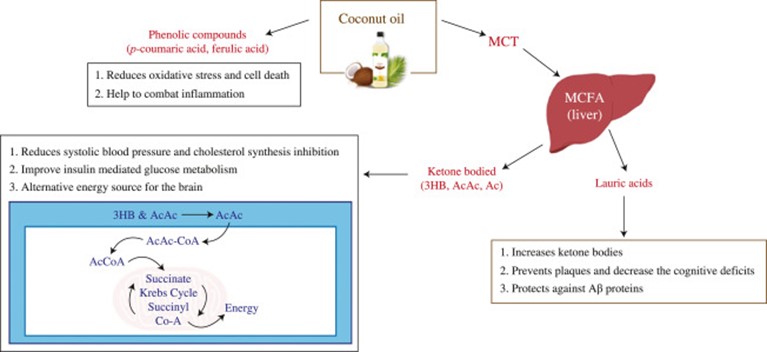
Fig. 4. Therapeutic effects of coconut oil as a treatment for AD.
In addition, tau phosphorylation is also responsible for the development of neurofibrillary tangles [2] which interferes with the transportation of nutrients within the neuron. As hyperphosphorylated tau proteins, disturbs the transport structure, starvation of neurons taken place, ultimately leading to cell death [19].
Generally, there are 100 trillion synapses in a healthy brain, permitting signals to transmit rapidly along with the neuronal circuits, developing the cellular basis of sensations, memories, emotions, thoughts, and skills [23]. Synaptic loss is another pathological characteristic that is strongly associated with cognitive impairments in AD [24]. Structural studies performed with the postmortem brain tissues of AD patients showed a significant depletion of synapse density compared to the age-matched control brain tissues mainly in hippocampus and cortex areas [25].
AD pathobiology affects microglia like nonneuronal cells as well. Microglia are a kind of immune system cells that activate with the development of Aβ and tau proteins [21]. The functionality of microglia cells is to eliminate these toxic proteins and the ubiquitous debris from dying cells. But at a certain tipping point, microglia are unable to function adequately, and eventually, chronic inflammation has taken place [23].
As a result of all these neurological detriments, nearly 80% of neurons especially in the hippocampal and cerebral cortex areas may die progressively, causing neuronal atrophy or shrinkage in those brain regions [26].
Mutation in genes for the APP or genes for presenilin 1 and 2 proteins can be a reason for a small percentage of Alzheimer’s incidents [18]. Besides, a form of apolipoprotein E, which is (APOE) ε4 allele, is found to be a greater genetic risk factor for both familial and sporadic types of AD [27]. Some researchers stated that this lipophilic apolipoprotein E has a strong association with the pathology of late-onset AD since it is the principal cholesterol carrier protein in the brain [28] and has the ability to affect the rate of disease progression [29]. Abnormal aggregation of Aβ and tau proteins, disruption of mitochondria, and cellular cytoskeleton are some potential risks that occurred with the presence of APOE ε4 [5]. In addition, this can contribute to lipid and cholesterol processing, inflammatory responses [30], cholinergic activity, and the proportion of neuronal cell death [27].
Many physiological processes are interrelated with the etiopathogenesis of AD. Among them, lipid metabolism, mitochondrial functions, oxidative stress, neurotransmitter function, and immune function are some crucial means that are altered with AD [31]. Corruption in the respiratory chain along with the mitochondrial dysfunction may lead to the accumulation of Aβ plaques whereas alterations in APP processing taking place [32].
Oxidative stress is the other prominent alteration, which can be expressed as the imbalance between the formation of reactive oxygen species and their revocation by defensive mechanisms [33]. The resulting chronic inflammation which is stimulated by oxidative stress would be a possible reason behind AD neuropathology. Oxidized proteins, lipid peroxidase, and glycated products are some intensive indicators that reveal the damage of oxidative stress and ultimate neurodegenerations [34]. On the other hand, decrement in antioxidant enzyme activity and surplus production of free radicals mediate the neurological impairments in AD [35].
Moreover, individuals who are with AD may come up with certain metabolic changes such as glucose hypometabolism which leads to inadequate glucose uptake and inefficient glycolysis subsequently developing gradual cognitive disabilities, due to the repression of GLUT1 transporter in the brain [36].
-
Risk factors enhancing AD
Many risk factors are associated with AD although the likelihood of occurrence of AD risk due to these remains uncertain. The risk of AD may be increased by a low level of education, obesity, severe head injury, diabetes, cerebrovascular disease, dietary patterns, behavioral patterns like smoking, and age [37].
Out of all these factors, age is considered as the most prominent risk factor for AD [38]. The protection of the young brain is done by several mechanisms along with higher levels of growth factors, better energy metabolism, and more efficient mechanisms for clearing misfolded proteins and repairing cells. Although the aggressive autosomal dominant AD mutations may not cause obvious deficits until the fourth or fifth decade of life, failure of the protective mechanisms might increase the risk of the development of AD [39].
As a result of this, 3% of people who are between age 65‒74, 17% of people in age 75‒84, and 32% of people in age 85 or older have AD [40]. Moreover, individuals who have a parent, or a sibling with AD are more likely to develop the disease than those who do not have a first-degree relative with AD. The estimated lifetime risk for AD is age 45 which is approximately one in five (20%) for women and 1 in 10 (10%) for men [23]. Not only that but also the prevalence of obesity, diabetes, and atherosclerosis are affected by age through metabolic or vascular mechanisms [41].
The transport of cholesterol from peripheral circulation into the brain is normally prevented by an intact blood-brain barrier that separates the peripheral and central regulation of [42]. However, when the integrity of this barrier is compromised by vascular injuries like a severe head injury or work, the regulated entry of cholesterol-carrying lipoproteins into the brain may be impaired. The consequences of vascular injuries on the blood-brain barrier and cholesterol entry into the brain are not entirely known but may result in increased accumulation of membrane cholesterol, setting up an adverse interaction between cholesterol and the pathology of AD [43].
There is enough emerging evidence about the link between cholesterol and AD [44] as it develops the pathogenic mechanisms by modulating the processing of APP [45]. Also APOE glycoprotein consists of very-low-density lipoproteins mainly (VLDL) [46]. All forms of fat including cholesterol, MUFA, and SFA are responsible for the formation of cholesteryl ester. The high dietary intake of SFA and cholesterol raise the low-density lipoproteins (LDLs), thus facilitating and accelerating the process of atherosclerosis [47].
An awareness of this AD and cholesterol relationship has fostered two therapeutic hypotheses; one proposing that high total cholesterol or LDL-cholesterol is a risk factor for AD and the other predicting that cholesterol-lowering strategies will have therapeutic value for AD [48]. One study has shown that the level of HDL cholesterol in the serum of AD patients is lower than that of controls and the decreased level is correlated with the severity of AD [49].
Present evidence indicates that dietary factors could affect the pathological process of AD. The development of obesity and metabolic syndrome is related to excessive consumption of red meats, refined sugars, high-fat foods, and refined grains that contain high concentrations of saturated and trans fatty acids [50]. Fatty acids, among all the food components, play indispensable roles in human nutrient demand and healthy regulation.
Fatty acids in CNS contribute to cell membrane composition, axonal growth [51] and function regulation, neuroinflammation response, and as an energy source [52]. FA compositions of natural foods vary as there are higher saturated fatty acids in meat and dairy products, while fruits and vegetables contain predominantly unsaturated FAs. Trans fatty acids are made either by the ruminal and intestinal bacterial metabolism or the hydrogenation of multiple unsaturated FAs from vegetable oils which are hypercholesterolemic and are linked to an adverse outcome with a high risk of cardiovascular diseases [53]. Therefore, the minimization of SFA and trans FA intake is recommended to lower the AD risk [29].
On the other hand, factors involved in the pathogenesis of AD include metabolic alterations such as insulin resistance and hyperglycemia, which are also hallmarks of type-2 diabetes mellitus [54]. The brain is one of the most energy-demanding organs relying on efficient ATP production via glycolysis, the TCA cycle, and oxidative phosphorylation [55]. However, glucose metabolism in AD brains is significantly impaired may well be due to oxidative modification, which often leads to decreased activity of the enzymes involved in glucose metabolism [56]. Altered glucose uptake is also observed in the brain of patients with mild cognitive impairment, considered an early stage of AD [57]. Further studies are needed to validate the impact of glucose uptake in the brain of mammalian models to predict the relationship with AD.
Impaired vascular health is another major risk factor for cognitive decline and interventions for cardiovascular risk may therefore improve cognitive health at the population level [58]. Other lifestyle-related factors, such as obesity, smoking, physical and mental inactivity, have been suggested to play a role in AD, and potential preventive measures related to these risk factors should be investigated [59]. Although AD is complex and mostly attributed to interactions between genetic factors and environmental factors; age, lifestyle, dietary habits, and behavioral patterns are also likely to contribute to the development of AD.
-
Impact of dietary supplementation on AD/ dietary approaches towards AD
The nutritional approach is considered as one of the promising strategies in order to slow, eliminate or halt the progression of AD around the world. As incorrect details lead to a false relationship between diet, risk factors, and prevention of AD, accurate assessment of usual dietary intake is crucial [60]. “Many nutritional supplements and dietary modifications may directly influence the pathological contributions of increased oxidative stress”, defects in mitochondrial dysfunction and cellular energy production, chronic inflammatory mechanisms, and even direct pathways to amyloid accumulation and neurofibrillary degeneration that contribute to the degenerative cascade in AD [1].
There are natural plant materials mainly like polyphenols, carotenoids, and vitamins with a wide range of biological effects, especially in the treatment of AD [61]. Among all, metabolic substrates, vitamins, omega-3-fatty acids, plant flavonoids, trace minerals, and antioxidants among others, are known as the main dietary constituents which downregulate the many pathological processes linked to the progression of AD, including aging and other risk factors [1].
Recently focusing on high consumption of carbohydrates and micronutrients from plant-based foods, moderate consumption of unsaturated fats (mostly from vegetable oils and fish), and low consumption of saturated and trans-fats, especially from animal-derived foods such as meat and dairy AD preventive diets have been introduced [60].
Mediterranean diet (MeDi), the Dietary Approaches to Stop Hypertension (DASH) diet, and the Mediterranean-DASH diet Intervention for Neurodegenerative Delay (MIND) diet are some examples of the above mentioned dietary types [62]. Overall, the MeDi provides abundant amounts of monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), fiber, and antioxidants (such as vitamin E), with limited amounts of saturated fatty acids and trans-fats [63]. From a nutritional perspective, the DASH diet provides high amounts of nutrients such as fiber, protein, potassium, magnesium, and calcium, while providing low amounts of saturated fat, total fat, and cholesterol [62]. The MIND diet was created to primarily capture the specific neuroprotective components of the MeDi and DASH diets [60].
Some findings have led to the use of antioxidant therapy including vitamin C, vitamin E, and selenium in the prevention of AD, which is found in vitamin C from citrus fruits and certain vegetables, and vitamin E from nuts, grains, and egg yolks [64].
On the other hand, vitamins like vitamin B, folate, vitamin B12, and vitamin B6, are of interest in AD prevention and treatment due to their roles in lowering plasma homocysteine levels.
Out of all the fatty acids, ω-3 PUFAs are considered substances with neuroprotective effects due to their roles in preventing inflammation, cerebrovascular and cardiovascular diseases, and the formation and aggregation of Aβ plaques. Higher intakes of ω-3 PUFA, particularly docosahexaenoic acid, have been associated with a reduced risk of AD [65].
The development of assessing food and beverage intake to facilitate the prevention of AD or diet-related chronic diseases is a major concern in the current society. Coconut oil is undoubtedly accepted as one of the compounds that are alternative therapeutic strategies of AD due to its unique fatty acid profile [66]. It has been stated that the coconut oil as a treatment for Aβplaques and both components found in coconut oil and metabolites including lauric acid (LA), ketone bodies (KBs), and medium-chain triglyceride (MCTs) have a potential mechanism of action relevant to AD [67].
-
Coconut oil and its composition
Coconut oil is extracted from the kernel of the mature coconuts of the coconut tree (Cocos nucifera) which belongs to the palm family (Family Arecaceae) [68]. This edible oil is extensively used in many tropical and subtropical countries for a variety of food and industrial purposes [69]. Due to the esteemed uses and beneficial characteristics of coconut oil, the global demand, as well as coconut oil production has been increasing over the past decade. According to the estimations, the world coconut oil consumption is around 3.5 MT/annum and that amount is accountable for 2.5% of global vegetable oil production [70]. Different types of coconut oils can be produced by using many processing methods. The most typically producing type is copra oil, which is obtained from the mechanical milling of the dried kernel [61]. Other than that, at present virgin coconut oil is rising in popularity with nutritional benefits, because it is extracted from the fresh kernel under mild conditions [71].
Coconut oil is characterized by 90% of saturated fatty acids, whereas the major fatty acid is lauric acid. Since lauric acid dominates the total fatty acid profile with a 35.25%‒52.48% proportion, coconut oil is also emphasized as a lauric oil [61]. In addition to the lauric acid, there are some other saturated fatty acids such as capric acid (7.00%), myristic acid (20.4%), palmitic acid (11.2%), stearic acid (2.6%), and arachidic acid (1.4%) [72].
Apart from these saturated fatty acids, there are monounsaturated and polyunsaturated fatty acids in relatively low amounts. Oleic acid is the most common monounsaturated fatty acid which has a 5.5% fraction in the total fatty acid profile of coconut oil [72]. Furthermore, linoleic acid and linolenic polyunsaturated fatty acids are there in 1.8% and 1.1% amounts respectively [72].
Generally, depending on the chain length of the fatty acid, they can be categorized as; short-chain (C2-C6), medium-chain (C6-C12), and long-chain (C14-C24) fatty acids [73]. Medium Chain Triglycerides (MCTs) are referred to as mixed triglycerides of saturated fatty acids, which are having a chain length of 6‒12 carbons [74]. Compared to the Long Chain Triglycerides (LCTs), MCTs are possessing distinct physical and chemical attributes, such as; small molecular size, remains in the liquid phase at room temperature, and low melting point [75]. MCTs are superabundant in coconut oil since the fatty acid profile dictates by medium-chain fatty acids (MCFA) [76].
Therefore, it can be suggested that diverse health benefits offered by coconut oil are credited to its MCFAs rich composition which corresponds to 64% of total fatty acids [73]. According to a comprehensive study, performed over 14 types of vegetable oils, coconut oil has the highest amount of MCFAs [77] and it reveals the uniqueness of coconut oil that exerts remarkable therapeutic properties [61].
-
Therapeutic role of coconut oil on AD
6.1. Metabolism of coconut oil MCT and AD pathogenesis
Diets containing MCTs are considered to be safe for human health due to their advantages over vascular disorders [78]. The contribution of MCT to lipid metabolism implies these benefits and it links the impact of MCT with AD pathology [65]. Therefore, the distinct characteristics in absorption, metabolism, and digestion of MCT are the distinguished reasons behind the reduction of AD risk factors.
Metabolism of MCFA, with the assistance of enzymes in saliva and gastric juices, requires a short time and moderate energy than the other fatty acids in diet which breaks down with pancreatic fat-digesting enzymes [79].
In the absorption pathways, the cholesterols and Long-chain fatty acids (LCFA), are combined with proteins and produce lipoproteins which are capable of entering the bloodstream through the lymphatic system while passing over the liver. During this circulation of lipoproteins, their fatty components may disperse and accumulate in body tissues while some of these fats coagulate on artery walls [80]. Thus, the risk for hypertension and cardiovascular diseases will be enhanced, ultimately resulting in an elevated susceptibility for AD [81]. In contrast, MCFAs are hydrolyzed by lingual lipase, available in the stomach, up to a certain extent and complete digestion takes place with the presence of pancreatic lipase in the intestinal lumen [82]. Therefore, MCFA, which resists esterification and binding, poorly contributes to the fat deposits, as they are directly absorbed to the portal vein from the intestine and directly sent to the liver [83].
On the outer mitochondrial membrane of liver mitochondria, the fatty acid metabolism is initiated, whereas the acyl-CoA synthetase enzyme catalyzes this process. At first, the particular fatty acid generates acyl-adenylate, and secondly that acyl adenylate is transformed into acyl-CoA and AMP [84].
MCFA has a specific pathway for the transportation of resulting acyl-CoA into the mitochondrial matrix, without depending on the carnitine transport system [85]. After transferring into mitochondria, medium-chain fatty acyl-CoA dehydrogenase enzyme can convert these medium-chain fatty acyl-CoA molecules into ketone bodies; most prominently acetoacetate and β-hydroxybutyrate. These ketone bodies are further metabolized, to produce H2O, CO2, and energy [66]. Instead of fat deposition, energy generated from the metabolism of MCFAs is efficiently converted into fuel for the utilization of organs and muscles [79]. This rise in fat oxidation and greater energy expenditure associated with the consumption of MCFAs instead of LCFAs helps in reducing the susceptibility of hyperlipidemia, hypertension, and obesity which are in turn the risk factors for AD [86].
This impact can be proved by the study performed with healthy subjects who received diets containing 10 g of MCT for 12 weeks and other similar subjects who received 10 g of LCT during the same period of time. According to the results of the study, subjects who received an MCT-rich diet achieved a significant decrease in body fat compared to the others who received an LCT-rich diet [49].
It is known that improvement in total and low-density lipoprotein cholesterol (LDL-C) is associated with the pathogenesis of cardiovascular diseases and in turn AD [73]. But the elevated levels of high-density lipoprotein cholesterols (HDL-C) are beneficial as they reduce the risk of hyperlipidemia by transporting phospholipids and cholesterols, out of the artery walls [87]. Lauric acid which is the major fatty acid in coconut oil supports the formation of HDL-C during its metabolism and it indicates the benefits of coconut oil on reducing susceptibility to cardiovascular complications. A randomized trial, performed with healthy volunteers who are supplemented with coconut oil for 8 continuous weeks, shows a significant improvement in HDL-C without changing the amounts of LDL-C and total cholesterol relative to the control [73].
Moreover, the depletion in the activity of the HMG-CoA reductase enzyme may decelerate the conversion of HMG-CoA protein to Mevalonate whereas the synthesis of cholesterol is inhibited. Lauric acid has the potential to retard the action of the HMG-CoA reductase enzyme and therefore it further denotes the efficacy of coconut oil on cholesterol synthesis inhibition and its therapeutic advantages over AD [87]. According to the results of a study that gives an MCT enriched diet during 12 weeks for an experimental group, cholesterol and triglyceride levels are slowly reduced while VLDL cholesterol content has a more pronounced deduction with the MCT diet [75].
6.2. MCFA and bile acid synthesis
The beneficial role of MCFA in lipid metabolism and control of some diseases like; cardiovascular diseases and AD are investigated in many studies while emphasizing the involvement of bile acid synthesis [88]. The amphipathic bile acids are simple end products of cholesterol metabolism and those are known as primary bile acids whereas secondary bile acids are produced by gut microbiota [89]. The relationship between MCFAs and the depressed pathology of AD can be explained by the role of MCFAs in reducing the serum cholesterol levels which is considered as a risk factor for AD. MCFAs engage in the serum cholesterol-lowering process through a partial increase in the excretion of bile acids and mostly this occurs via the decline of absorption in the small intestines [88].
This impact can be verified further by an experimental trial that involves feeding of tauroursodeoxycholic acid (TUDCA) which is a bile acid derivative. According to the results of this trial, it was found that feeding with TUDCA reduces Aβ aggregates, minimizes tau phosphorylation and neuroinflammation while improving the memory of the subjects [90].
6.3. Association between coconut oil and insulin response
Obesity, CVD, disrupted cholesterol metabolism, insulin resistance, type 2 diabetes, and hypertension like health issues are considered as risk factors for AD [91]. As, insulin exerts multiple effects in the brain, including neurotrophic, neuromodulatory, and neuroendocrine actions, the presence of insulin and the insulin receptor (IR) in the CNS suggest that the brain is a target for the action of insulin [92]. Moreover, insulin and glucose metabolism support the idea that the metabolic alterations of diabetes mellitus type 2 are strongly associated with the development of AD as they induce neuronal death due to oxidative stress and inflammatory response affecting cognitive processes [54].
Although there is no effective treatment has found for AD, recent studies have shown that the incorporation of coconut oil in diets can reduce the risk factors, because of the major disruption in insulin function that happens early in the AD pathogenesis [93]. A study proved that, compared to other oils, coconut oil in the diet enhances insulin action and improves binding affinity [68]. The effect of MCFA on insulin secretion depends on its chain length. Capric acid (C10) and lauric acid were observed to display the most potent effects on insulin secretion, out of all the MCFA [94]. Insulin resistance, correlating with triglyceride accumulation in skeletal muscle, can be induced (in rats) by a diet high in saturated fatty acids. In contrast, MCFA intake was observed to significantly stimulate basal insulin secretion and insulin sensitivity in rats and a mouse islet model, which could be due to improved ATP production via mitochondrial β-oxidation, and greater signal transduction for the exocytosis of insulin [66].
In an in vitro study that compared LCFA and MCFA effects in myotubes, it has been found that MCFA-treated cells displayed less lipid accumulation, and MCFA increased the intrinsic respiratory capacity of mitochondria without increasing oxidative stress [51].
Major compounds in coconut oil that are believed to reduce IR are polyphenolic compounds and fatty acids, although not all studies agree with this. This indicates that further research is required to understand the metabolism and effects of different MCFA [54].
6.4. Ketone bodies as an alternative energy source for the AD brain
Ketones or ketone bodies like AcAc, 3-hydroxybutyrate (3HB), and acetone inside the body, are made out of non-carbohydrate fuel sources like MCT or MCFA. Acetone is a breakdown product of AcAc, while AcAc and 3HB are used for energy production [59].
The human brain primarily depends on glucose as fatty acids cannot pass the blood-brain barrier [95]. Therefore, the energy homeostasis of the brain is maintained by utilizing alternative fuels such as monocarboxylic acids, lactate, and ketones, especially in the case of an insufficient amount of glucose. The ketone bodies are produced from stored fat during starvation, as well as from MCFAs which are found in different dietary sources like coconut oil which is considered as nature’s richest source of MCTs [69]. The use of MCTs in diets has increased the level of β-hydroxybutyric acid (β-OHB) and had a positive impact on the rollback of memory reaction in AD pathogenesis [61].
A study done in 2004 took MCTs from coconut oil and put them into a drink that was given to Alzheimer’s patients while a control group was given a placebo. A significant increment in levels of the ketone body β-OHB was observed after 90 min of the treatment. When cognitive tests were administered, higher ketone values were associated with greater improvement in paragraph recall with MCT treatment relative to placebo across all subjects [68,96].
Furthermore, in the brain cells, mitochondria act as tiny power plants to transform glucose into energy for cell activities. During AD pathogenesis, cerebral glucose metabolism seems to suffer as mitochondria become less able to absorb and use glucose. Ketones which are broken down from MCT, act as a glucose substitute offering the necessary simple fuel for the dysfunctional mitochondria in the brain cells to use for energy [71]. Therefore ketone bodies are considered to be broadly neuroprotective and have been reported to lessen amyloid toxicity [69].
When talking about the blood-brain barrier [BBB], it is a brain endothelial structure of the fully differentiated neurovascular system that protects the brain from foreign substances. It is very difficult to develop an effective neurotherapeutic for AD, as more than 98% of all small-molecule drugs, and approximately 100% of all large-molecule drugs or genes, cannot cross the BBB. However, according to the literature D–β-3hydroxy-butyrate ketone body, which is formed out of MCFA, crosses the BBB and enters the mitochondria in brain cells [79]. Here AcAc converts to acetyl-CoA, which enters into the Krebs cycle for the energy metabolism. In one in vivo study with mice, the capacity of caprylic acid, which is a constituent of coconut oil, has been demonstrated as an anticonvulsant and a neuroprotectant [79].
Even though the published studies on animals and people seem to identify that ketones are safe, it is important to check with a physician before starting an over-the-counter ketone food, such as virgin coconut oil or MCT oil. These dietary patterns should be aligned with individual differences regarding cardiovascular or liver conditions, insulin regulation, and tolerance and sensitivity to foods and medicines, such as gastrointestinal upset [97]. Concerns about acidosis, hypocalcemia, hyperlipidemia, insulin resistance, and carcinogenesis have been identified according to the research and the large amounts of calories in ketone food [71]. May lead to an unhealthy weight gain too. On the other hand, people with an allergy to any form of coconut, palm kernel oil, milk, or soy should avoid coconut products altogether. Ketogenic diets along with high-protein diets may cause possible kidney damage due to high levels of N excretion during protein metabolism [79].
6.5. Neuroprotective properties of other coconut oil components
Nutraceuticals and dietary interventions for the prevention of AD have been a major concern as a treatment for AD due to the absence of FDA-approved therapeutics and the failures of recent clinical trials [67]. Despite differences existing among various dietary inventions, the higher intake of fruits, vegetables, fish, nuts, and legumes like food commodities which are rich in antioxidants, trace minerals, flavonoids, vitamins, metabolic substrates, and modulators, and a lower intake of high-fat dairy, and sweets seemed to be associated with lower odds of cognitive deficits or reduced risk of AD [1].
As coconut oil is one of the compounds that have been reported with anecdotal effects, some claims propose coconut oil as a treatment for AD pathogenesis due to its constituents like lauric acid, ketone bodies, medium-chain triglyceride, and phenolic compounds [69].
6.5.1. Phenolic compounds
Phenolic compounds are a group of chemical compounds that are classified as secondary metabolites of plants that exhibit several bioactive properties including antioxidant activity [33]. Substances with antioxidant activity have a high potential to scavenge free radicals which directly links with the development of AD due to oxidative stress [56]. The hydroxyl group of phenolic compounds may be able to reduce the toxicity of the Alzheimer’s Ab peptide [79]. Coconut oil has a high percentage of phenolic acids, like p-coumaric acid, ferulic acid, caffeic acid, and catechin acids, which are sometimes also referred to as polyphenols [67].
Coconut oil is known to have a therapeutic value in AD pathogenesis through targeting oxidative stress. VCO polyphenol pre-treatment was suggested to reduce oxidant-induced oxidative stress and cell death by restoring to near-normal the levels of glutathione, as well as glutathione reductase, glutathione peroxidase, and catalase activities in the cells according to different cell studies [98]. In a rat model of AD, it has observed a reduction in oxidative stress and in the expression of the inflammasome-associated gene, NLRP3, which had been induced via Aβ and the high-fat diet. This was carried out through the addition of VCO to a high-fat diet [99]. Some in vitro studies it has shown that CO has a protective effect on the survival of cortical neurons exposed to Aβ and suppressed the mitochondrial alterations which were induced by Aβ in the control experiment [100].
Maltolyl p-coumarate, an ester of p-coumaric acid which is found in CO, has been reported to decrease cognitive deficits in scopolamine injected rats and Aβ42-infused rats [101].
The CO-derived phenolic compound ferulic acid has been observed to lower cortical Aβ levels in an AD transgenic mouse model. Ferulic acid also possesses anti-inflammatory properties, in the mice injected with Aβ42 by reducing glial fibrillary acidic protein and IL-1β after chronic administration of ferulic acid [102]. The processing methods such as wet processing and dry processing play a vital role in modulating phenolic compounds present in Coconut oil [73]. However, all these constituents need further research to check their efficacies.
6.5.2. Sterols
Phytosterols are known as the structural and functional analogs of cholesterol that are synthesized in plant organisms. Sterols received greater attention recently due to their anti-tumor and hypocholesterolemic effects contributing to lower the level of total cholesterol and LDL fraction, and also inhibit platelet aggregation [73]. Cholesterol promotes the formation of plaques, which are composed of particularly Aβproteins which deposit at nerve cells within the brain causing AD [38].
The total content of sterols in coconut oil is about 70 mg/100 g and several authors in their studies showed 3 different sterol fractions in coconut oil as campesterol, stigmasterol, and β-sitosterol with an average content are 7.20%, 12.30%, and 38.97% [61]. The potentiality of these sterols in lowering the risk factors of AD by influencing the metabolic processes should be further investigated.
6.5.3. Tocopherols
Tocopherols are lipid-soluble natural antioxidants found in most vegetable oils. Vitamin E is a prominent example of tocopherols with protective properties against unsaturated fatty acids which decreases the susceptibility of unfavorable oxidation processes [1].
It is estimated that coconut oil contains 4.20 mg/100 g of tocopherols. The dominant fraction of tocopherols in coconut oil is α-tocopherol (α-T), with a share estimated at 0.20%‒1.82%. Other tocopherols such as γ, β, and Δ-tocopherol (γ, β, and Δ-T) in coconut oil are 0.12%, 0.25%, and 0.39%. In coconut oil, there are also fractions of tocotrienols, dominated by the content of α-tocotrienol (α-T3) in quantities ranging from 1.09% to 3.0%. The content of such tocotrienols as γ, β and Δ (γ, β, and Δ-T3) is 0.33%‒0.64%, 0‒0.17%, and 0‒0.10%, respectively [61].
Despite the amount of tocopherols, they may inhibit oxidative damage which is a key factor in the pathology of AD [56].
Although many studies have suggested that coconut oil contains many antioxidants with the potential to reduce the development of AD pathology, due to the inconsistencies in the data, it is suggested that further research needs to be undertaken before broadly advocating the use of coconut oil as a dietary intervention.
-
Different techniques of coconut oil extraction, compositional changes, and their impact on AD
Extraction of coconut oil can be carried out using different methods, which may give varying compositional properties for the coconut oils. The most common techniques to extract oil out from the coconut fruit are described below while highlighting the variation of their polyphenol contents and antioxidant capacity.
The total phenolic content (TPC) in coconut oil is highly affected by different processing techniques used in the industry [107]. When comparing the TPC of hot extracted coconut oil, the value is exceeding the TPC of coconut oil extracted from the cold extraction method. The reason behind this variation is, the polar nature of the phenolic compounds and their tendency to dissolve in the aqueous phase of the coconut milk emulsion which is favored by the hot temperatures as the concentration of phenolic compounds is elevated with the evaporation of water from the emulsion [103]. Therefore, the mild conditions applied in the cold extraction method are not capable of having such a greater amount of phenolic substance in the extracted oil. Even though it was found that high temperatures may lead to destruction in some phenolic compounds [108]. The experimental results in Table 1 prove that hot extraction is better in incorporating phenolic substances into the coconut oil than the cold extraction method.
Table 1. Different extraction methods and their impact on total phenolic content of coconut oil
| Method | Process (with special process conditions) | Impact on Total phenolic content / antioxidant capacity |
| Cold extraction | The aqueous layer is discarded from the chilled coconut milk, at 10 °C to obtain the lipid block and allow it to dissolve at 30 °C [103] | Total phenolic content ∼ 0.066 mg/g [103] Phenolic acid composition: 18.01% Gallic acid 2.36% Ferulic acid [61] |
| Hot extraction | Break the emulsion in coconut milk by heating to 100‒120 °C and water is evaporated. At last, oil is decanted from the deposits [103] | Total phenolic content ∼ 0.449 mg/g [103] Phenolic acid composition: 25.29% Gallic acid 12.83% Ferulic acid [61] |
| Natural fermentation | Coconut milk is added with hot water and allows it to settle for 72 h within the closed container [104]. | Total phenolic Content ∼ 12.54 mg/g [104] Phenolic acid composition: 5.09% Ferulic acid 2.08% Vanillic acid [61] Antioxidant activity 19.7% [105]. |
| Chilling & centrifugation | Coconut milk is subjected to centrifugation at 3220 r/min for 10 min in order to separate the coconut cream. The cream is chilled at 0 °C for 6 h and thawed at room temperature. Then, centrifugation is applied at 4000 r/min for 60 min under room temperature, to extract the oil [105]. | Total phenolic content ∼ 1.16 mg/g [104] Antioxidant activity 23.51% [105] |
| Direct micro expelling-sun-dried method. | The sun-dried kernel is pressed mechanically under high pressure [104]. | Total phenolic content ∼ 8.57 mg/g [106] |
According to the values presented, the fermentation method of coconut oil extraction exerts the highest amount of phenolic compounds. During the fermentation method, the oil layer is settled separately from the aqueous phase and prolonged contact with the phenolic solution, and this separated oil layer might explain the higher concentration of phenolic substances in the extracted oil [107].
Direct micro expelling which is involved with a sun-drying process has a relatively smaller number of phenolic compounds than the fermented coconut oil. This can occur as most of the drying processes are destroying some types of phenolic compounds in the coconut [109].
In general, the increase in phenolic content may lead to accelerating antioxidant activity [107]. However, the table demonstrated that the chilling & centrifugation method of coconut oil extraction has a very low TPC value, while its antioxidant activity of 23.51% is exceeding the fermented coconut oil which has the highest phenolic concentration. This interesting observation can be justified by the preservation of thermally unstable antioxidant compounds during the cold extraction condition involved in the chilling & centrifugation method [104].
Refining is another practice employed in coconut oil production and its respective TPC value ranges within 0.06‒0.14 mg/g [110], while the antioxidant activity of refined coconut oil lays around 9.73% which is much lower than the previously described oil types [105]. The exposure to excessive heat during the refined oil extraction process will be responsible for this impact [109].
Therefore, virgin coconut oils extracted from the above-mentioned methods are better options for the extraction of coconut oil compared to refined coconut oil due to its higher phenolic antioxidant capacity [105].
Even though coconut oil is suggested as a therapeutic treatment for AD, as a source enriched with antioxidants such as polyphenols, it will render more promising benefits by using virgin coconut instead of refined coconut oil. Moreover, the experimental results may suggest that virgin coconut oil extracted from the fermentation method is ideal for this therapeutic treatment.
-
Limitations
Lack of cure or effective treatments, as well as undiscovered scientific, medical, and clinical advances, have caused a growing population around the world to deal with AD and other related dementias. Nutritional interventions with promising agents like vitamins, energy substrates, flavonoids, lipids, modified diets with antioxidants, metabolic-enhancers, immune-modulators, and direct disease-modifying agents appear to be a safe approach to prevent AD due to the cost-effectiveness, ease of administration, and social acceptability.
However, further studies are needed to determine the potential adverse effects versus benefits of these nutritional interventions based on the differences of individuals, and the types of progressive dementias. Coconut oil as a therapeutic treatment for AD has been an emerging popular dietary modification although no definitive evidence is available.
Coconut oil was criticized and consumers were made to believe that coconut oil has deleterious effects on health as it would clog arteries due to the saturated fatty acids till very recently. But with recent evidence claiming the positive health benefits owing to the presence of MCTs, this tide has turned in a positive way. However, some findings are suggesting that despite coconut being a promising dietary intervention, incorporation of coconut oil in diets in the long run and its influence on neuronal function and survival, as well as cardiovascular effects remains unknown.
At the same time, ketone bodies that are supposed to influence b-amyloid levels and their protection against AD requires further study. Especially the dosage of ketones, the duration of activation, and the optimum conditions also need to be investigated. Though coconut oil and MCT oil deliver ketones as a substitute for glucose in the cerebral glucose metabolism as mitochondria diminish during AD more studies are needed to determine if ketone foods act as mitochondrial medicine to improve cognitive function or contribute to further mitochondrial dysfunction in the long-term consumption.
On the other hand, more research needs to be conducted to quantify the yield of ketones from coconut oil under different extraction methods and their ability to cross the BBB, to establish the efficacy. For example, the extraction method used to obtain VCO appears to affect the quality of coconut oil and may directly affect the efficacy, which means if some specific extraction methods are essential to achieve efficacy, only particular preparations would confer benefit.
After concluding the facts, although the nutritional components of coconut are well applicable and accepted, due to the inconsistency of data, further research supported by large cohort clinical data for a long run needs to be undertaken before advocating the use of coconut oil as a therapeutic treatment for AD. However, the anecdotal evidence and positive findings on coconut oil highlight its potentiality as a compound of interest for further investigation as a dietary intervention for AD pathogenesis.
-
Conclusion
Nutritional supplements and dietary modifications may directly influence the pathological contributions of increased oxidative stress, defects in mitochondrial dysfunction and cellular energy production, chronic inflammatory mechanisms, and direct pathways to amyloid accumulation and neurofibrillary degeneration which are the main physiological processes interrelated with the etiopathogenesis of AD. It has been stated that the components found in coconut oil including lauric acid (LA), ketone bodies (KBs), and MCTs have a potential mechanism of action relevant to AD, with the recent recognition and evidence.
The therapeutic role of coconut oil can be discussed under different pathways. Dissimilar to most other dietary fats that are high in long-chain fatty acids, coconut oil contains MCFA. MCFA are unique in that they are easily absorbed and metabolized by the liver, resisting esterification and binding, and poorly contributing to the fat deposits. The evidence is mounting to support, that this process is certainly beneficial not only in the treatment of AD, but also in obesity, dyslipidemia, elevated LDL, insulin resistance, and hypertension which are risk factors for cardiovascular diseases and type 2 diabetes. On the other hand, MCFAs engage in the serum cholesterol-lowering process through a partial increase in the excretion of bile acids and mostly this occurs via the decline of absorption in the small intestines.
Moreover, ketone bodies like AcAc, 3HB, and acetone inside the body, are made out of non-carbohydrate fuel sources like MCT or MCFA. They are a significant alternative energy source in the brain and may be beneficial to people developing or already with memory impairment. Although more than 98% of all small-molecule drugs and approximately 100% of all large-molecule drugs or genes cannot cross the BBB, the D–β-3hydroxy-butyrate ketone body crosses the BBB and enters the mitochondria in brain cells according to the literature. However, much epidemiological and intervention study evidence is needed to warrant further investigations on ketone bodies to establish efficacy. In addition, phenolic compounds found in coconut oil may aid in preventing the aggregation of Aβ peptide, with its anti-inflammatory, anti-oxidative, and anti-amyloidogenic properties potentially inhibiting a key step in the pathogenesis of AD. The experimental results may suggest that antioxidant capacity depends on the techniques to extract oil out from the coconut fruits and virgin coconut oil extracted from the fermentation method is ideal as a therapeutic treatment.
Coconut oil was criticized mentioning that it has deleterious effects on health as it would clog arteries due to the saturated fatty acids till very recently, but with recent evidence claiming the positive health benefits owing to the presence of MCTs. Coconut oil should be emphasized as a treatment or a preventive measure for AD which has highly nutritious functional properties. However, due to the inconsistencies of any peer-reviewed large cohort clinical data for the long run, it is suggested that further research needs to be undertaken before broadly advocating the use of coconut oil as a dietary intervention.
Reference
See original article

