HOW STATISTICAL DECEPTION CREATED THE APPEARANCE THAT STATINS ARE SAFE AND EFFECTIVE IN PRIMARY AND SECONDARY PREVENTION OF CARDIOVASCULAR DISEASE
David M Diamond and Uffe Ravnskov, Medical Research Service, Veterans Hospital, Tampa, 33612 FL, USA Department of Psychology, Center for Preclinical and Clinical Research on PTSD, University of South Florida, Tampa, 33620 FL, USA Department of Molecular Pharmacology and Physiology, Center for Preclinical and Clinical Research on PTSD, University of South Florida, Tampa, 33620 FL, USA Independent Researcher, Magle Stora Kyrkogata 9, 22350 Lund, Sweden *Author for correspondence: ddiamond@usf.edu
Expert Review Clinical Pharmacology. Early online, 1–10 (2015)
Ronald Peters, MD, MPH Commentary:
Contrary to Big Pharma’s media campaign, statin drugs do not prevent coronary heart disease, but they do increase the risk for musculoskeletal disorders, cancer, fatigue, diabetes, and cognitive impairment, especially in the elderly. Cholesterol is an essential molecule for health. It is used for tissue repair as well as estrogen and vitamin D production. Cholesterol is also required to make bile in the liver so you can absorb fat soluble vitamins such as vitamin E and K. As Dr. Diamond shows in the research article, Big Pharma uses “relative risk reduction” to make the statin drugs appear to prevent heart disease. Relative risk compares statin with some other (bad) treatment and statin is better. “Absolute risk reduction” is key and from this perspective statins are no better than placebo in preventing heart disease. (See the graphic below) Also, a whole food, plant-based diet has been shown in solid research to prevent and reverse coronary artery disease with lots of side effects, including better digestion, energy levels and moods.
We have provided a critical assessment of research on the reduction of cholesterol levels by statin treatment to reduce cardiovascular disease. Our opinion is that although statins are effective at reducing cholesterol levels, they have failed to substantially improve cardiovascular outcomes. We have described the deceptive approach statin advocates have deployed to create the appearance that cholesterol reduction results in an impressive reduction in cardiovascular disease outcomes through their use of a statistical tool called relative risk reduction (RRR), a method which amplifies the trivial beneficial effects of statins. We have also described how the directors of the clinical trials have succeeded in minimizing the significance of the numerous adverse effects of statin treatment.
The reputed role of high serum cholesterol as an etiological factor in cardiovascular disease (CVD) has been a source of controversy and debate for decades. This debate has been described as a war between the advocates, who view high cholesterol as a causal factor in coronary heart disease (CHD) [1,2], against the skeptics, who consider cholesterol a vital component of cell metabolism [3–10]. The advocates’ main argument is based on the presence of cholesterol in atherosclerotic tissue, and studies demonstrating an association between high levels of serum cholesterol and CHD. Skeptics, by contrast, have emphasized that a comprehensive review of the literature reveals that there is a lack of evidence of a causal link between cholesterol and CHD (coronary heart disease}.Indeed, an absence of an association between cholesterol levels and the degree of atherosclerosis in unselected people was originally described in 1936 [11], a finding which has been confirmed in numerous contemporary studies. The fact is that older adults with low levels of cholesterol are just as atherosclerotic as those with high levels [12]. That high cholesterol is not a risk factor for CHD has been documented in many studies on a broad range of individuals, including women, Canadian men, Swedes, Maoris, elderly people and patients with CHD [3,4,8,13,14].
Although the extensive research demonstrating that CHD occurs independent of cholesterol levels is incompatible with Hill’s criteria for causality, the advocates are winning the war. Today millions of healthy people are on statins, which are drugs that reduce cholesterol levels via inhibition of HMG-CoA reductase. Moreover, the number of healthy people on statins will increase considerably if the new guidelines from the American College of Cardiology and the American Heart Association are followed [15].
Despite the many contradictory findings, the advocates have praised statins as ‘miracle drugs’ which are ‘the best anti-atherosclerotic insurance’ [16], as well as ‘the most powerful inventions to prevent cardiovascular events’ [17]. They have also promoted the view that ‘there is no longer any doubt about the benefit and safety’ of reducing cholesterol levels [18]. The skeptics have acknowledged that statin treatment has been shown to reduce coronary events, but close inspection reveals that the benefit is much less impressive than clinicians and the general public have been told and that it must be because of other mechanisms than cholesterol reduction [8,19,20].
In this review, we have evaluated findings in a representative subset of the statin trials and case–control studies. We have concluded that the beneficial effects on CVD are actually miniscule and that their adverse effects are more common than is generally known. We have described how the appearance of the impressive effects has been accomplished through the exploitation of a statistical anomaly named relative risk, and by designing and interpreting the studies in a way as to minimize the appearance of adverse effects. Overall, our goal in this review is to explain how the war on cholesterol has been fought by advocates that have used statistical deception to create the appearance that statins are wonder drugs, when the reality is that their trivial benefit is more than offset by their adverse effects.
How statistical deception created the appearance of statins as ‘miracle drugs’
Resolution of the issue as to how statin effects on coronary events have been misrepresented requires an appreciation of the terminology used in clinical research, in general, as well as a thorough analysis of the raw data in the statin trials.
The statistical terms we will focus on are relative and absolute risk, relative and absolute risk reduction (ARR) and the number needed to treat (NNT). To illustrate the use of these terms in clinical research, consider a 5-year trial that includes 2000 healthy, middle-aged men. The aim of the trial is to see if a statin can prevent heart disease. Half of the participants are administered the statin and the other half a placebo. In most clinical trials, we find that during a period of 5 years about 2% of all healthy, middle-aged men experience a nonfatal myocardial infarction (MI). Consequently, at the end of our hypothetical trial, 2% of the placebo-treated men and 1% of the statin-treated men suffered an MI. Statin treatment, therefore, has been of benefit to 1% of the treated participants. Thus, the ARR, which quantifies how effective a treatment is on the population at risk, was one percentage point, and the NNT was 100, resulting in only 1 of 100 people benefiting from the treatment. Put another way, the chance of not suffering from an MI during the 5-year period without treatment was 98% and by taking a statin drug every day it increased by 1 percentage point to 99%.
When it comes to presenting the findings of this hypothetical trial to healthcare workers and the public, the directors of this trial do not think people would be impressed by a mere 1% point improvement. Therefore, instead of using the ARR they present the benefit in terms of relative risk reduction (RRR). The RRR is a derivative of the ARR in which the difference in disease outcomes in two groups is expressed as a ratio. Hence, using RRR, the directors can state that statin treatment reduced the incidence of heart disease by 50%, because 1 is 50% of2.
Representative examples of statistical deception in statin trial data presentation
In this section, we have focused on three clinical trials, which illustrate how statistical deception has magnified the unimpressive effects of statin treatment in the medical literature and in the media using RRR.
JUPITER
The first one is the JUPITER trial [21], in which rosuvastatin (Crestor) or placebo was administered to 17,802 healthy people with elevated C-reactive protein, but with no prior history of CHD or elevated cholesterol levels. The primary outcome was the occurrence of a major cardiovascular event, defined as nonfatal MI, nonfatal stroke, hospitalization for unstable angina, arterial revascularization or death from cardiovascular causes.
The trial was stopped after a median follow-up of 1.9 years. The number of subjects with a primary endpoint was 251 (2.8%) in the control group and 142 (1.6%) in the rosuvastatin group. The difference in endpoint rate of 2.8% vs 1.6% yields an ARR of 1.2 percentage points and an NNT of 83. The benefit with regards to the number of fatal and nonfatal heart attacks was even smaller. There were only 68 (0.76%) vs 31 (0.35%) events, respectively, resulting in, an ARR of 0.41 percentage points and an NNT of 244. This means that regarding fatal and nonfatal CHD, less than one-half of 1% of the treated population (0.41%) benefited from rosuvastatin treatment, and 244 people needed to be treated to prevent a single fatal or nonfatal heart attack. Despite this meager effect, in the media the benefit was stated as ‘more than 50% avoided a fatal heart attack’, because 0.41 is 54% of 0.76.
Thus, the public and healthcare workers were informed of a 54% reduction of heart attacks when the actual effect in the treated population was a reduction of less than 1 percentage point. Moreover, the ARR of 0.41 percentage points was the combination of fatal and nonfatal heart attacks. There was little attention paid to the fact that more people had died from a heart attack in the treatment group. Even experienced researchers may have overlooked this finding because the figures were not explicitly stated in the report. One needs to subtract the number of nonfatal CHD from the number of ‘any MI’ to see that there were 11 fatal heart attacks in the treatment group, but only six in the control group.
Despite the miniscule effects of rosuvastatin reported in the publication, in the media the JUPITER findings were presented as very impressive. In an article in Forbes Magazine, John Kastelein, a co-author of the study, proclaimed: ‘It’s spectacular . . . We finally have strong data’ that a statin prevents a first heart attack. This triumphant declaration of victory in the war on cholesterol convinced an US FDA advisory panel to recommend Crestor treatment for people with elevated C-reactive protein levels and normal levels of cholesterol.
According to a table in the JUPITER report there was no difference between the numbers of serious adverse effects between the two groups. However, in the rosuvastatin group there were 270 new cases of diabetes, but only 216 in the control group (3% vs 2.4%; p < 0.01). Unlike beneficial effects, which the authors amplified in the magnitude of its appearance using RRR, the significant effect of new onset diabetes by Crestor treatment was expressed only in the ARR form.
An objective assessment of the JUPITER findings should therefore be conveyed to potential patients in the following manner: ‘Your chance to avoid a nonfatal heart attack during the next 2 years is about 97% without treatment, but you can increase it to about 98% by taking a Crestor every day. However, you will not prolong your life and there is a risk you may develop diabetes, not to mention other serious adverse effects’ (which we shall describe in a later section).
ASCOT-LLA
The second trial we have focused on is the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOTLLA) [22]. The reason is that the findings have been promoted in advertisements to the public and medical professionals as representative of the robust effects of CHD risk reduction with statin treatment in primary prevention.
This trial included 10,305 individuals with hypertension. In addition, all of them had at least three of the following risk factors: Type 2 diabetes, left ventricular hypertrophy, peripheral arterial disease, previous stroke or transient ischemic attack, or smoking. Half of them received 10 mg atorvastatin, half of them a placebo and the primary endpoint was nonfatal and fatal CHD.
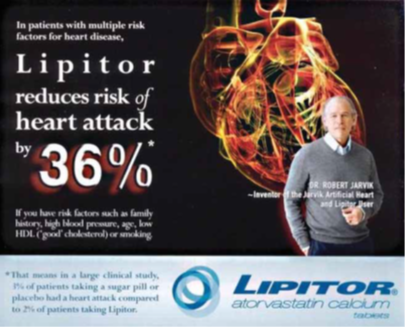
| Figure 1. An advertisement for Lipitor which emphasizes the relative risk reduction of a heart attack (36%), while minimizing the appearance of the absolute risk data (3 vs 2%) in the lower section. |
The trial was planned to continue for 5 years, but the authors found the preliminary findings so impressive that the study was terminated at 3.3 years. The reason was that at that time ‘cholesterol lowering with atorvastatin 10 mg conferred a 36% reduction in fatal CHD and nonfatal MI compared with placebo’.
However, the benefit was actually unimpressive. In the placebo group, 3% suffered a heart attack vs 1.9% in the atorvastatin group. Thus, the ARR was only 1.1 percentage points, which is 36% of 3. Moreover, there was no significant benefit in subgroups of patients at high risk of CHD, including those with diabetes, left-ventricular hypertrophy and previous vascular disease or for patients aged 60 years or younger, for those without renal dysfunction and for individuals with metabolic syndrome. For women there were no benefits at all. Indeed, there was a trend for worse, albeit non-significant, effects. Finally, there was no effect on either cardiovascular or non-cardiovascular mortality.
Why was ASCOT stopped prematurely after 3.3 years when there was a notable absence of benefit in most measures. Because the primary basis of the premature termination was ‘the impressive 36% reduction of fatal CHD and nonfatal MI’, which also became the focal point of advertisements (FIGURE 1).
It is a useful exercise to illustrate how the RRR of 36% was derived. In FIGURE 2, we have graphed the findings (from their Table 3) to demonstrate the small ARR produced by drug treatment. The data are expressed in terms of events with 100% reflecting the absence of a cardiovascular event for each group. The figure illustrates the following points:
- If a patient with a risk profile of subjects in ASCOT asks a physician about the likelihood that he or she will not experience an MI or a fatal coronary event without treatment, the figure provides this information. The first column reveals that 97%, virtually all of the placebo-treated subjects, did not have a nonfatal MI or die of CHD.
- The next series of categories illustrates the almost complete absence of other major coronary events in placebo-treated subjects. The asterisks in FIGURE 1 represent statistically significant effects which are based on the miniscule differences in the rate of events between the drug and placebo-treated groups. As can be seen, the difference in outcomes with drug treatment is only about one percentage point for all measures.
The British Heart Protection Study
The British Heart Protection Study (HPS) included more than 20,000 adults aged 40–80 years with prior evidence of CVD and/or diabetes. Half of the study population was allocated simvastatin (40 mg/day) and the other half a placebo for the 5-year study.
The findings were discussed in an accompanying editorial [23] which praised the effects of cholesterol lowering in this trial, as well as in a press release with the headline: ‘LIFE-SAVER: World’s largest cholesterol-lowering trial reveals massive benefits for high-risk patients.’ The Lancet editorial stated that ‘the implications of these findings are profound.’ Professor Rory Collins, the Director of the study, was emphatic with his praise
in a news report by stating ‘This is a stunning result, with massive public health implications. We’ve found that cholesterol lowering treatment . . . can prevent strokes as well as heart attacks.’ He further stated that the study ‘provides the first direct evidence that cholesterol lowering therapy cuts the risk of heart attacks and strokes by at least one-third.’ In the trial report, it was stated that there was an ‘18% reduction’ in the
coronary mortality rate, and an ‘extreme 38% proportional reduction in the incidence rate of first nonfatal MI’. Overall, the investigators reported ‘a 27% . . . reduction in the incidence rate of nonfatal MI or coronary death.’
We will now look at the ARR instead of the RRR. In the simvastatin group, 781 (7.6%) had died because of CVD, in the placebo group the number was 937 (9.1%). Thus, the ARR was only 1.5 percentage points (9.1–7.6) and the NNT was 67.
A largely undiscussed feature of the study, which is common to statin trials, in general, was that 26% of all eligible subjects withdrew from the study after being on simvastatin for 1 month before the formal initiation of the study (the run-in period). The reason for their withdrawal was not provided, but a likely explanation may be that they did not tolerate the adverse effects of the drug. Thus, any study that has a period in which
subjects with adverse events may withdraw before formal study initiation has an inherent bias against providing a representation of the actual rate of adverse events. Hence, the rate of statin adverse effects cannot be determined from such studies.
Systematic bias minimizing adverse effects of statins
We have discussed how the magnitude of the beneficial effects of statin treatment is meager, typically in the range of a 1–2 percentage point reduction in the rate of coronary events. Nevertheless, at a global level, a reduction of coronary events and death in 2% of the population could make a substantial difference if statins did not have any adverse health effects. However, the adverse effects are substantial, including an increased rate of cancer, cataracts, diabetes, cognitive impairment and musculoskeletal disorders [24–30]. Whereas the benefits of statins are routinely reported as relative risk, adverse effects are always expressed in terms of absolute risk. In the following, we have focused only on three serious adverse effects of statins: cancer, myopathy and disorders of the CNS and how they have been downplayed in importance.
Cancer
Numerous statin trials have reported an increase in the incidence of cancer. In four of them, the increase was statistically significant. Here, we shall analyze a subset of these findings in detail.
The CARE trial was a secondary-preventive trial including 4159 patients (576 women and 3583 men) with MI and average cholesterol levels [31]. Half of the patients were administered 40 mg pravastatin, half of them placebo. After 5 years treatment, 24 (1.15%) had died because of CHD in the treatment group and 38 (1.83%) in the placebo group, resulting in an ARR of 0.68 percentage points.
The most serious adverse event was breast cancer, which occurred in 12 of the women (4.2%) in the pravastatin group but in only one of the women (0.34%) in the placebo group. Although the difference in the incidence between the groups was statistically significant (p = 0.002), the authors dismissed the increased risk by stating: ‘There is no known potential biologic basis. . .the totality of evidence suggests that these findings
in the CARE trial could be an anomaly and may be best interpreted in the context of the trial’s very low event rates and statistical testing of many adverse events.’
We disagree with these authors regarding a lack of evidence for a mechanistic link between statins, or low cholesterol in general, and cancer. Research indicates that lipoproteins actively participate in immune system functioning by binding to and inactivating all kinds of microorganisms and their toxic products [32]. Moreover, there is a well-established role of viruses in cancer development [33], and it is well-known that reduced levels of cholesterol are associated with a greater incidence of viral infection and cancer, for instance hepatitis B and liver cancer [34]. Furthermore, at least nine cohort studies have shown that low cholesterol measured 10–30 years previously is a risk actor for cancer later in life [35]. Moreover, several case–control studies of cancer patients and healthy controls have shown that the cancer patients had been using statins significantly more often than the control subjects [35]. In accordance, at least two non-statin cholesterol-lowering trials have also reported a statistically significant excess of cancer cases in the treatment groups [36,37].
Other statin trials have resulted in cancer, as well. For example, PROSPER was a large trial involving 5804 men and women aged 70–82 years with a history of, or risk factors for, vascular disease. Half of them were given pravastatin, the other half a placebo [38]. At follow-up 3.2 years later, they wrote in the abstract that mortality from heart disease had fallen by 24%. However, according to one of the tables, 4.2% had died
from a heart attack in the control group and 3.3% in the treatment group, thus with an ARR of only 0.9 percentage points. The small cardiovascular benefit was neutralized by a substantial number of patients who had died from cancer. There were 28 fewer deaths from heart disease in the pravastatin group, but 24 more deaths from cancer. If we include nonfatal cancer in the calculation, the cancer difference was statistically
significant; 199 in the control group and 245 in the pravastatin group (p = 0.02). Furthermore, the cancer difference between the two groups increased year over year. Despite the statistically significant effects of pravastatin treatment on newly diagnosed cancer, the conclusion from the authors was that ‘the most likely explanation is that the imbalance in cancer rates in PROSPER was a chance finding, which could in part have been driven by the recruitment of individuals with occult disease.’
To further minimize the significance of the findings, the authors counted the number of new cancers in all previous pravastatin trials and found that taken together there was no significant increase. But in this calculation they did not include the number of individuals with skin cancer, and they did not mention that in the previous trials the participants were 20–25 years younger. PROSPER was a particularly important
and unique trial because it focused on statin treatment of elderly people only. Cancer is a frequent finding at postmortem of older people whose death is attributed to another cause. However, the cancer is often dormant or it grows so slowly that it never becomes a problem during their lifetime – unless the growth is stimulated by an exogenous factor, for instance by carcinogenic chemicals.
If statin treatment or low cholesterol is cancer-provoking, as it has been shown in animal experiments [39], cancer is likely to show up first in people with the highest risk of cancer, for instance in elderly people. There are also great differences between the incubation periods for different cancers. Those that are easy to detect are also those that will appear the earliest. To exclude skin cancer from the trial reports is therefore to
introduce a serious bias. In the two first simvastatin trials, 4S and Heart Protection Study (HPS) [40,41], more patients in the treatment groups were diagnosed with non-melanoma skin cancer. Although these figures appeared in the tables, the authors did not mention this finding in the text, possibly because the differences were not statistically significant, but if the data from both trials are combined, the statin-cancer association is significant (256/12 454 vs 208/12 459; p < 0.028).
Another statin trial where cancer occurred more often in the treatment group is SEAS [42]. In that trial, 1873 patients with various degrees of aortic stenosis and with a mean total cholesterol of 222 mg% (5.7 meq/l) were included. Half of them were treated with simvastatin and ezetimibe, the other half with a placebo. Except for ischemic events, no significant benefit was seen for any of the clinical outcomes after 4.3 years
treatment. However, cancer appeared in 105 patients (11.1%) in the treatment group, but only in 70 patients (7.5%) in the control group, a statistically significant effect (p < 0.01). The authors noted the increased incidence of cancer in the treated group, but they added that ‘as long-term statin therapy has not
been associated with an increased risk of cancer,’ they concluded that ‘the observed difference in cancer rates in the study may have been the result of chance’ and nothing was mentioned about the cancer finding in the abstract.
Finally, most statin trials are terminated within 2–5 years, a period which is too short to see most cancers develop. It is notable in this context that one long-term (ten-year) case control study of several thousand women demonstrated that there was a doubling of the risk of ductal and lobular breast cancer among those who had used statins for more than 10 years (odds ratio 2.00; 1.26–3.17) [29].
Whether the statins are inherently carcinogenic is an open question. In any case, there is strong evidence that low cholesterol, in general, and statin use are both associated with an increased risk of cancer [35].
Myopathy
It is widely accepted that myopathy is the commonest adverse effect from statin treatment and it is seen most often in women and elderly people [43–46]. For instance, Sinzinger et al. [45] have reported that muscular weakness and pain occur in one out of four statin-treated patients who exercise regularly. They also noted that 17 out of 22 professional athletes with familial hypercholesterolemia treated with statins stopped because of that particular side effect [46].
Golomb et al. [43] performed a randomized controlled trial that included 1016 healthy men and women with high LDLC. Here, the participants were divided into three groups that were given 20 mg simvastatin, 40 mg pravastatin or placebo. After 6 months treatment, 40% of the women on statin treatment experienced adverse effects on energy or exertional fatigue.
However, in almost all reports from the statin trials it is said that muscle damage occurs in less than 1% of treated subjects. To reach that number, the authors have only recorded muscular damage in patients with high creatine kinase (CK), and high CK is defined as a value that is 10-times higher than the normal upper limit at two successive determinations. A relevant question is what happens after many years of statin treatment with the muscles of people whose CK is ‘only’ nine times higher than normal? Furthermore, people on statins may have muscular problems although their CK is normal [47], and even people on statins without any symptoms may have microscopic evidence of muscular damage [48].
Another way to minimize the muscular symptoms is to separate them into numerous categories. According to the FDA Adverse Event Reporting System adverse muscular symptoms are recorded in 11 categories (muscle disorder, myopathy, muscle tightness, musculoskeletal stiffness, myalgia, muscular weakness, muscle cramp, muscle enzyme, muscle fatigue, muscle necrosis and muscle spasm). In most of them, a low incidence of adverse effects is reported, which disperses the total number of adverse event reports across many subtypes of pathology. Taken together, however, the total number of myopathy-related adverse events is substantial. Finally, muscular side effects are not benign phenomena; they may in particular have a deleterious effect on elderly people, because the least expensive and the least risky way to prevent heart disease is regular exercise.
CNS pathology, including mood & cognitive disorders
There is much evidence that low cholesterol is associated with diseases in the CNS. For example, in a meta-analysis of cholesterol-lowering trials, Muldoon et al. [49] found a statistically significant increase in the number of deaths from accidents, suicide or violence in the treatment groups. Although fewer people died because of a heart attack, more died because of neurological causes. The authors also noted that low blood cholesterol levels are seen more often in criminals, in people with diagnoses of violent or aggressive-conduct disorders, in homicidal offenders with histories of violence and suicide attempts related to alcohol, and in people with poorly internalized social norms and low self-control.
Muldoon et al. finding has been confirmed by other authors. In a review published 4 years later, Boston et al. [50] concluded that lowering cholesterol levels have been associated with an increase in violent deaths in cardiovascular primary prevention studies, and that altered cholesterol levels had been reported in relation to other psychiatric disorders. Finally, Asellus et al. [51] found that in patients with serum cholesterol below the median, the correlation between exposure to violence as a child and adult violence was significant.
Whether the aggressive state causes low cholesterol or low cholesterol causes increased aggression was addressed by Muldoon’s group in an experiment with monkeys. They noted that a reduction of their plasma cholesterol increased their tendency to engage in impulsive or violent behavior [52]. In a comment on this article, Horrobin [53], the former editor of Medical Hypotheses, wrote that the most serious consequence of cholesterol-lowering measures may be invisible. That is, if low cholesterol levels cause violence and depression, then intervention to reduce cholesterol on a large scale could lead to a general shift to more violent patterns of behavior by statin users, a symptom that has not been investigated in any trial.
A low serum cholesterol level has also been found to serve as a biological marker of major depression and suicidal behavior, whereas high cholesterol is protective [54–57]. In a study by Davison and Kaplan [58], the incidence of suicidal ideation among adults with mood disorders was more than 2.5-times greater in those taking statins. Moreover, several studies have shown that low cholesterol is associated with lower cognition and Alzheimer’s disease and that high cholesterol is protective [59,60]. These observations of reduced brain functioning with statins have been supported by Evans and Golomb. In a study of 143 patients with memory loss or other cognitive problems associated with statin therapy, they reported that 90% of them improved, sometimes within days, of statin discontinuation [27]. In a study by Padala et al. [61], 18 older statin-treated subjects with Alzheimer’s disease were asked to stop their statin treatment. Twelve weeks later, their performance on several cognition tests had improved significantly and after having started the treatment again, their performance on the tests worsened significantly.
A strong argument for the view that statin treatment may cause adverse CNS effects is a study by Sahebzamani et al. [62] of adverse events from statin treatment reported to the FDA. They found that there was a disproportionately greater incidence of adverse cognitive events reported by patients who were treated with lipophilic statins.
If low cholesterol predisposes an individual to develop cerebral abnormalities, then peripheral nerves may be targets of statin-induced pathology, as well. This issue was addressed by Gaist et al. [63] in a study of 465,000 people in Denmark. The authors asked all patients who had polyneuropathy of unknown cause how many were on statin treatment compared with the general population in the county. They calculated that the risk for definite polyneuropathy was 16-times higher for current statin users than for non-users (OR: 16.1; CI: 5.7–45.4), and even higher for those who had used statins for more than 2 years (OR: 26.4; CI: 7.8–45.4).
One problem is that if mentioned at all on the drug labels, these cerebrospinal adverse effects of statins are characterized as rare, perhaps because they are classified into many different subgroups. According to FDA Adverse Event Reporting System, adverse effects from cerebrospinal dysfunctions are classified in 23 separate reaction terms (suicidal attempt, suicidal ideation, suicidal behavior, aphasia, balance disorders, coordination abnormal, amyotrophic lateral sclerosis, amnesia, memory impairment, transient global amnesia, cognitive, confusional state, irritability, paranoia, disorientation, dementia, depression, depressed mood, neuropathy, pain in extremity, Guillain–Barre syndrome, ALS and multiple sclerosis). The incidence of statin related side effects in the many different subcategories is present at a low rate, but if all of them were to be combined the total number of adverse events may be substantial.
Finally, with regard to overall cerebral functioning with statin treatment, a cautionary note is deserved. In most cases, the adverse effects appear gradually, over several months after the start of the treatment. Impaired cognition may be falsely attributed to advanced age or early dementia; both the patients and their doctors may be unaware that the cognitive symptoms may result from a slowly developing impairment of brain functioning as a result of insufficient brain cholesterol synthesis. The importance of cholesterol contributing to optimal levels of CNS functioning parallels findings we described previously regarding higher levels of cholesterol related to a reduced incidence of cancer. Thus, multiple lines of research indicate that low levels of cholesterol, in general, and statins, in particular, are associated with neuropathology and poorer cognitive functioning
Expert commentary & five-year view
We have documented that the presentation of statin trial findings can be characterized as a deceptive strategy in which negligible benefits of statin treatment have been amplified with the use of relative risk statistics, and that serious adverse effects are either ignored or explained away as a chance occurrences. Moreover, the authors of these studies have presented the rate of adverse events in terms of absolute risk, which, compared to relative risk, minimizes the appearance of their magnitudes.
We are compelled to address the issue of financial conflicts of interest as a potential source of bias, as well. An example is the cholesterol guidelines published in 2012 by the Cholesterol Treatment Trialists’ (CTT) Collaborators [64]. This group is part of the Clinical Trials Service Unit in Oxford, which has received hundreds of millions of pounds over recent years to conduct research on behalf of the pharmaceutical companies.Their conclusion was that ‘in individuals with 5-year risk of major vascular events lower than 10%, each 1 mmol/l reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1000 over 5 years. This benefit greatly exceeds any known hazards of statin therapy.’
However, no clinical trial has shown any association between the degree of cholesterol lowering and the outcome using absolute risk statistics. But if the CTT recommendations are followed, almost all adults will be taking statins for the rest of their lives.
Despite the promotion of statins largely through the exclusive use of relative risk statistics, there is rising skepticism against statin treatment in primary prevention [65–67], and as we have shown, there is little evidence that statins provide a substantial benefit in secondary prevention, as well. Almost all trials have found that the NNT to avoid a fatal CHD in a 5-year period is at least 50, and in most of them it is over 100. Moreover, as documented by many independent researchers, and as we have reviewed here, the adverse effects of statins are more serious and far more common than is typically reported in the trial publications. The low rate of reporting of adverse events is based on multiple factors we have reviewed here, including the routine inclusion of an initial run-in phase in which statin intolerant individuals are removed from a study before formal initiation. Moreover, subdividing adverse events into many different categories, as in the reports for myopathy and cerebral disorders, can make it more difficult to identify subtle, but consistent, statin-related pathologies. Even if only 10% of statin-treated individuals suffer from adverse effects, a conservative estimate to be sure, this means that millions of healthy people all over the world will become patients who will experience adverse drug effects without any benefit.
The selective control over data handling, including detailed information regarding adverse events, has led independent researchers to request open access to the clinical trial data, which the drug companies have denied. If the statin trials have been performed appropriately and objectively, why do the study directors restrict access to their findings?
Recently, new guidelines for statin treatment were published by the American College of Cardiology and the American Heart Association with an even more aggressive risk threshold including treatment for almost all elderly people and diabetics [68]. These guidelines were discussed in an opinion article in New Your Times by John Abramson, MD, a lecturer at Harvard Medical School, and Rita Redberg, MD, the editor of JAMA Internal Medicine. They asserted that the new guidelines would result in recommendations for statin treatment to a ‘vastly expanded class of healthy Americans’.
There are other reasons to be wary of conflicts of interest by authors of the new guidelines; for instance eight of the 15 panelists had extensive ties to the pharmaceutical industry [69] and according to Angell [70], the former editor of JAMA, and Gøtzsche [71], head of Nordic Cochrane, several of the major drug companies have paid billions of dollars to settle civil and criminal charges of fraud, illegal marketing and bribery,. We therefore welcome more medical journals to follow new rules introduced by the British Medical Journal, in which ‘clinical education articles will be authored by experts without financial ties to industry’ [72].
It is tempting to be cynical and pessimistic about the future. There is a great appeal to the public to take a pill that offers the promise of a longer life and to live heart attack free. The reality, however, is that statins actually produce only small beneficial effects on CVD outcomes, and their adverse effects are far more substantial than is generally known. Nevertheless, if the pharmaceutical industry continues to expand its control over medical education, research and the media, then 5 years from now most adults, as well as children with elevated cholesterol levels [73], will be on a statin.
We prefer to consider a more enlightened and optimistic future. With sufficient awareness of the deception underlying the promotion of statin treatment, clinicians may opt for better alternatives. Diabetics and obese people should be educated as to the great value of a low carbohydrate diet for normalizing all of their biomarkers of cardiovascular risk, including obesity [74–76]; all people should be informed about the hazards of consuming food with partially hydrogenated fats [77,78] and about the benefits associated with exercise, stress reduction and the consumption of foods high in saturated fats [79–82].
Financial & competing interests disclosure
D Diamond was supported by a Career Scientist Award from the US Department of Veterans Affairs. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript
References
- Steinberg D. The cholesterol controversy is over. Why did it take so long? Circulation 1989;80:1070-8
- Steinberg D. In celebration of the 100th anniversary of the lipid hypothesis of atherosclerosis. J Lipid Res 2013;54:2946-9 3. Pinckney ER, Pinckney C. The Cholesterol Controversy. Sherbourne Press; Los Angeles: 1975
- Smith RL. The Cholesterol Conspiracy. Warren H. Green; St. Louis, Missoury: 1991
- Ravnskov U. An elevated serum cholesterol level is secondary, not causal, in coronary heart disease. Med Hypotheses 1991;36:238-41
- Berger M. The cholesterol non-consensus. Methodological difficulties in the interpretation of epidemiological studies. Bibl Nutr Dieta 1992;49:125-30
- Gurr ML. Dietary lipids and coronary heart disease: old evidence, new perspective. Prog Lipid Res 1992;31:195-243
- Ravnskov U. A hypothesis out-of-date: the diet-heart idea. J Clin Epidemiol 2002;55:1057-63
- Grimes DS. An epidemic of coronary heart disease. QJM 2012;105:509-18 10. Marshall TM. New insights into the statin-cholesterol controversy. J Am Phys Surg 2014;19:42-6
- Lande KE, Sperry WM. Human atherosclerosis in relation to cholesterol content of blood serum. Arch Pathol 1936;22:301-13
- Ravnskov U. Is atherosclerosis caused by high cholesterol? QJM 2002;95:397-403
- Rosch PJ. Cholesterol does not cause coronary heart disease in contrast to stress. Scand Cardiovasc J 2008;42:244-
- Rosch PJ. Cholesterol does not cause coronary heart disease in contrast to stress. Scand Cardiovasc J 2008;42:244-9
- Schersten T, Rosch PJ, Arfors KE, et al. The cholesterol hypothesis: time for the obituary? Scand Cardiovasc J 2011;45:322-3
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 2014;129(25 Suppl 2):S1-45
- Roberts WC. The underused miracle drugs: the statin drugs are to atherosclerosis what penicillin was to infectious disease. Am J Cardiol 1996;78:377-8
- Jeger R, Dieterle T. Statins: have we found the Holy Grail? Swiss Med Weekly 2012;142:w13515
- Oliver M, Poole-Wilson P, Shepherd J, et al. Lower patients’ cholesterol now. BMJ 1995;310:1280-1
- Lindholm LH, Samuelsson O. What are the odds at ASCOT today? Lancet 2003;361: 1144-5
- Thompson A, Temple NJ. The case for statins: has it really been made? J R Soc Med 2004;97:461-4
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-207
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac OutcomesTrial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149-58
- Yusuf S. Two decades of progress in preventing vascular disease. Lancet 2002;360:2-3
- Foody J. Statin use associated with increased risk of cataract, myopathy, liver dysfunction and acute renal failure with varying numbers needed to harm. Evid Based Med 2010;15:187-8
- Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci 2012;89:1165-71
- Preiss D, Sattar N. Statins and the risk of new-onset diabetes: a review of recent evidence. Curr Opin Lipidol 2011;22:460-6
- Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy 2009;29: 800-11
- Goldstein MR, Mascitelli L. Do statins decrease cardiovascular disease at the expense of increasing cancer? Int J Cardiol 2009;133:254-5
- McDougall JA, Malone KE, Daling JR, et al. Long-term statin use and risk of ductal and lobular breast cancer among women 55 to 74 years of age. Cancer Epidemiol Biomarkers Prev 2013;22: 1529-37
- Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340:c2197
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med 1996;335:1001-9
- Ravnskov U, McCully KM. Vulnerable plaque formation from obstruction of vasa Review Diamond & Ravnskov doi: vasorum by homocysteinylated and oxidized lipoprotein aggregates complexed with microbial remnants and LDL autoantibodies. Ann Clin Lab Sci 2009;39: 3-16
- Read SA, Douglas MW. Virus induced inflammation and cancer development. Cancer Lett 2014;345:174-81
- Chen Z, Keech A, Collins R, et al. Prolonged infection with hepatitis B virus and association between low blood cholesterol concentration and liver cancer. BMJ 1993;306:890-4
- Ravnskov U, Rosch PJ, McCully KS. The statin-low cholesterol-cancer conundrum. QJM 2012;105:383-8
- Dayton S, Pearce ML, Hashimoto S, et al. A controlled clinical trial of a diet high in un-saturated fat in preventing complications of atherosclerosis. Circulation 1969; 40(suppl II):1-63
- Oliver MF. Cholesterol-lowering and cancer in the prevention of cardiovascular disease. QJM 2010;103:202
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomized controlled trial. Lancet 2002;360:1623-30
- Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA 1996;275:55-60
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002;360:7-22
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383-9
- Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343-56
- Golomb BA, Evans MA, Dimsdale JE, et al. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med 2012;172: 1180-2
- Golomb BA. Statins and activity: proceed with caution. JAMA Intern Med 2014;174: 12 0-2
- Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol 2002;40:163-71
- Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 2004;57:525-8
- Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002;137:581-5
- Draeger A, Monastyrskaya K, Mohaupt M, et al. Statin therapy induces ultrastructural damage in skeletal muscle in patients without myalgia. J Pathol 2006;210:94-102
- Muldoon MF, Manuck SB, Matthew HA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ 1990;301:309-14
- Boston PF, Dursun SM, Reveley MA. Cholesterol and mental disorder. Br J Psychiatry 1996;169:682-9
- Asellus P, Nordstro¨m P, Nordstro¨m AL, et al. Cholesterol and the “Cycle of Violence” in attempted suicide. Psychiatry Res 2014;215:646-50
- Kaplan JR, Muldoon MF, Manuck SB, et al. Assessing the observed relationship between low cholesterol and violence-related mortality. Implications for suicide risk. Ann N Y Acad Sci 1997;836:57-80
- Horrobin D. Personal view. Lowered cholesterol concentrations and mortality. BMJ 1990;301:55
- Agargu¨n MY, Algu¨n E, Sekerog˘lu R. Low cholesterol level in patients with panic disorder: the association with major depression. J Affect Disord 1998;50:29-32
- Kim JM, Stewart R, Shin IS, et al. Low cholesterol, cognitive function and Alzheimer s disease in a community population with cognitive impairment. J Nutr Health Aging 2002;6:320-3
- Ozer OA, Kutanis¸ R, Agargun MY. Serum lipid levels, suicidality, and panic disorder. Compr Psych 2004;45:95-8
- VilibicM, JukicV, Pand zic-Sakoman M, et al. Association between total serum cholesterol and depression, aggression, and suicidal ideations in war veterans with posttraumatic stress disorder: a cross-sectional study. Croat Med J 2014;55:520-9
- Davison KM, Kaplan BJ. Lipophilic statin use and suicidal ideation in a sample of adults with mood disorders. Crisis 2014;35:278-82
- Kim YK, Lee HJ, Kim JY, et al. Low serum cholesterol is correlated to suicidality in a Korean sample. Acta Psychiatr Scand 2002;105:141-8
- Hoyer S, Riederer P. Alzheimer disease—no target for statin treatment. Neurochem Res 2007;32:695-706
- Padala KP, Padala PR, McNeilly DP, et al. The effect of HMG-CoA reductase inhibitors on cognition in patients with Alzheimer’s dementia: a prospective withdrawal and rechallenge pilot study. Am J Geriatr Pharmacother 2012;10:296-302
- Sahebzamani FM, Munro CL, Marroquin OC, et al. Examination of the FDA warning for statins and cognitive dysfunction. J Pharmacovigil 2014;2:4-12
- Gaist D, Jeppesen U, Andersen M, et al. Statins and risk of polyneuropathy: a case-control study. Neurology 2002;58:1333-7
- Cholesterol Treatment Trialists (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581-90
- Sultan S, Hynes N. The ugly side of statins. Systemic appraisal of the contemporary un-known unknowns. OJ Endocr Metab Dis 2013;3:doi:10.4236/ojemd.2013.33025 66. Spence D. Bad medicine: statins. BMJ 2013;346:f3566
- McCartney M. Medicine and the media. Statins for all? BMJ 2012;345:e6044
- Stone NJ, Robinson J, Lichtenstein AH, et al. American college of cardiology/American heart association task force on practice guidelines. 2013 ACC/ AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 6(25 Pt B):2889-934
- Lenzer J. Majority of panelists on controversial new cholesterol guideline have current or recent ties to drug manufacturers. BMJ 2013;347:f6989
- Angell M. The truth about the drug companies: How they deceive us and what to do about it. Random House; NY: 2004
- Gøtzsche P. Deadly medicines and organised crime: How big pharma has corrupted healthcare. Radcliff Publishing Ltd; London: 2013
- Chew M, Brizzel C, Abbasi K, et al.n Medical journals and industry ties. BMJ 2014;349:g7197
- de Ferranti S, Ludwig DS. Storm over statins–the controversy surrounding pharmacologic treatment of children. N Engl J Med 2008;359:1309-12
- Volek JS, Fernandez ML, Feinman RD, et al. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res 2008;47:307-18
- Dashti HM, Mathew TC, Khadada M, et al. Beneficial effects of ketogenic diet in obese diabetic subjects. Mol Cell Biochem 2007;302:249-56
- Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 2015;31:1-13
- Kummerow FA. The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis 2009;205: 458-65
- Bendsen NT, Christensen R, Bartels EM, et al. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: a systematic review and meta-analysis of cohort studies. Eur J Clin Nutr 2011;65:773-83
- Feinman RD. Saturated fat and health: recent advances in research. Lipids 2010; 45(10):891-2
- Siri-Tarino PW, Sun Q, Hu FB, et al. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 2010;91:502-9
- Ravnskov U, Diamond D, Karatay MC, et al. No scientific support for linking dietary saturated fat to CHD. Br J Nutr 2012;107:455-7
- Ravnskov U, Dinicolantonio JJ, Harcombe Z, et al. The questionable benefits of exchanging saturated fat with polyunsaturated fat. Mayo Clin Proc 2014;89:451-3 Review Diamond & Ravnskov doi:





