The Marine Pilot Study 2020: Integrating Complementary and Alternative Medicine in the Treatment of Subconcussive and Concussive Brain Trauma.
Mark L. Gordon1, Alison M. Gordon-Gregg2, and Alicja B. Poleszak3. (2024) 1,2Millennium Health Centers, Inc. Neuroregeneration Division. Magnolia, Texas 77354. USA. 3Ageless Health, Traumatic Brain Injury Division, Chicago, Illinois, 60126.
Dr Peters’ Comments
If you have had head trauma with loss of consciousness (Concussive Brain Trauma), or, without loss of consciousness (Subconcussive) and you are having depression, anxiety, PTSD, loss of memory or any type of cognitive impairment, you may have chronic neuroinflammation which can be reversed. This article reviews a new path to wellness using natural nutrient therapies. Please call if you have questions.
Abstract
: The incidence of traumatic brain injury (TBI) in the United States remains alarmingly high despite ongoing educational efforts and advancements in safety measures. In 2017 alone, the Centers for Disease Control and Prevention (CDC) reported nearly 2.87 million TBI incidents, affecting a population of over 40 million Americans. Many individuals with TBI suffer from debilitating neuropsychiatric conditions such as depression and anxiety, contributing to an increased risk of suicide particularly within military and civilian populations. TBI was implicated in 61,131 deaths in 2017, comprising 2.2% of total deaths that year, with a significant proportion categorized as intentional injuries. Current treatment approaches utilizing psychotropic medication and psychotherapy have shown limited effectiveness, underscored by rising suicide rates among TBI patients. Research focus is shifting towards understanding the causal mechanisms underlying TBI-associated neuropsychiatric disorders, notably post-traumatic stress syndrome (PTSS or PTSD). Recent findings suggest a role for fractalkine, a neuronal chemokine, in modulating neuroinflammatory responses that may accelerate neurodegeneration and contribute to cognitive decline and psychiatric symptoms following TBI. Addressing these complexities is critical for advancing effective therapeutic strategies and improving outcomes for TBI patients.
(Key Words: Cytokines, Fractalkine, neurosteroids, N-Acetylcysteine, tocopherols, eicosanoids, pyrroloquinoline quinone, ubiquinone, thiamine, riboflavin, methylcobalamin, docosahexaenoic acid, ascorbic palmitate, epigallocatechin gallate, reactive oxygen species (ROS), nitric oxide (NO), and peroxynitrite (PN).)
Introduction
Traumatic brain injury (TBI) remains a significant public health concern, impacting individuals across diverse settings from military combat zones to recreational sports fields. The pathophysiological consequences of TBI are complex and multifaceted, initiated by traumatic insults that trigger immunological responses leading to the release of inflammatory cytokines such as interleukin-1 (IL-1), IL- 1b, IL-6, and tumor necrosis factor-alpha (TNF-alpha) (1). These cytokines play pivotal roles in initiating a cascade of events that include oxidative stress in the brain (2), disruption of biochemical pathways (3), breakdown of the blood-brain barrier (BBB) (4), neurotransmitter dysregulation (5), and impairment of hypothalamic-pituitary neurosteroid regulation (6), ultimately contributing to cognitive deficits (7) and the development of various neuropsychiatric disorders (8).
Efforts to identify definitive biomarkers for TBI have been ongoing, with markers such as S100B, glial fibrillary acidic protein (GFAP), neuron-specific enolase (NSE), myelin basic protein (MBP), Tau protein, and Amyloid β extensively studied (9). However, while these biomarkers confirm neurological damage post-injury, they fall short in elucidating the specific neurochemical alterations crucial for guiding targeted treatment approaches (10). Consequently, the development of effective therapeutic strategies has been hindered by the inability to predict individualized treatment responses based solely on these markers (11).
Historically, pharmaceutical interventions aimed at mitigating TBI symptoms have faced significant challenges, often yielding inconclusive or detrimental outcomes in clinical trials (12). The lack of substantial therapeutic efficacy underscores the urgency for novel approaches that address the underlying biochemical disruptions implicated in post-TBI mental health sequelae.
This article focuses on elucidating the biochemical mechanisms precipitated by both subconcussive and concussive injuries, which contribute to altered mental health outcomes. Additionally, it explores thescientific rationale supporting the application of nutraceutical interventions that target inflammation and mitochondrial dysfunction. Specifically, this paper reviews findings from a recent pilot study conducted within an active military setting, evaluating the efficacy of a defined nutraceutical intervention in ameliorating TBI-associated symptoms.
By comprehensively examining the underlying pathophysiology and exploring promising therapeutic avenues, this study aims to contribute to a deeper understanding of TBI mechanisms and to inform future research and clinical practice aimed at improving outcomes for affected individuals.
The Influence of Neuroinflammation on Mental Health
Neuroinflammation is a complex process that can be triggered by trauma, infection, or stress and is crucial for defending the central nervous system (CNS) (13). However, its profound impact on neurochemistry and overall brain function, particularly in influencing neuropsychiatric conditions, cannot be overstated (14).
One of the primary consequences of neuroinflammation is the alteration in neurochemistry. This disruption affects the delicate balance of neurotransmitters, neuromodulators, and other critical signaling molecules essential for neuronal communication and regulation. Neurotransmitters such as glutamate, GABA, dopamine, serotonin, and others play pivotal roles in controlling mood, cognition, and behavior through synaptic transmission and neuronal excitability (15).
During neuroinflammation, there is an increased release of pro-inflammatory cytokines, chemokines, and immune mediators. These substances disturb the neurochemical balance by, for instance, promoting excessive glutamate release and impairing its reuptake. This imbalance can lead to excitotoxicity, a process characterized by heightened glutamate signaling and subsequent neuronal damage, which is implicated in various neurodegenerative disorders and acute brain injuries (16).
Furthermore, neuroinflammation alters the availability and function of neurotransmitter receptors. Activation of microglia and astrocytes during this process can reduce the expression of key receptors like the N-methyl-D-aspartate receptor (NMDAR), thereby impairing synaptic plasticity. Such disruptions contribute significantly to cognitive deficits observed in neuroinflammatory conditions (17).
Additionally, neuroinflammation affects neurotransmitter synthesis and metabolism. It can influence the activity of enzymes crucial for dopamine, serotonin, and other neurotransmitters, leading to imbalances associated with neuropsychiatric symptoms such as depression, anxiety, and cognitive impairments (18).
Moreover, neuroinflammation disrupts neuromodulators like brain-derived neurotrophic factor (BDNF), essential for synaptic plasticity, neuronal survival, and maintaining neuronal networks. Dysregulation of BDNF signaling due to neuroinflammation is strongly linked to the pathophysiology of psychiatric and neurodegenerative disorders (19).
Taken together, neuroinflammation profoundly affects neurochemistry by disturbing the delicate balance of neurotransmitters, neuromodulators, and their receptors. These disruptions contribute significantly to neuronal dysfunction, excitotoxicity, and the onset of various neuropsychiatric symptoms. Understanding these neurochemical alterations is crucial for developing targeted therapeutic strategies aimed at restoring neurotransmitter balance and alleviating the detrimental effects of neuroinflammation on neuropsychiatric conditions.
[A more in-depth discussion on this topic is in reference (8), “Neuroinflammation, the road to neuropsychiatric illnesses (2023).” ]
Mood Disorders
Neuroinflammation has been strongly implicated in the pathophysiology of mood disorders such as major depressive disorder (MDD) and bipolar disorder. Studies have consistently demonstrated increased levels of pro-inflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), in the blood and cerebrospinal fluid of individuals with depression (20). These inflammatory mediators can impact neurotransmitter systems, such as serotonin and dopamine, and disrupt neural circuits involved in mood regulation. Furthermore, chronic neuroinflammation can lead to structural and functional changes in key brain regions implicated in depression, such as the prefrontal cortex and hippocampus (21).
Post-traumatic stress disorder
PTSD is a psychiatric condition that can occur in individuals who have experienced or witnessed a traumatic event, such as combat, assault, or natural disasters. The chronic activation of the stress response and the associated release of stress hormones can trigger an inflammatory response within the brain (22). This neuroinflammatory response involves the activation of microglia, the brain’s resident immune cells, and the release of pro-inflammatory cytokines and chemokines. These inflammatory mediators can disrupt normal neural circuitry, particularly in regions involved in emotional processing and stress regulation, such as the amygdala, prefrontal cortex, and hippocampus (23). Neuroinflammation-induced alterations in neurotransmitter systems, such as serotonin and glutamate, may further contribute to the emotional dysregulation and memory disturbances observed in individuals with PTSD.
Migraines and Headaches
Neuroinflammation plays a pivotal role in the pathophysiology of migraines, influencing both the initiation and progression of this debilitating neurological disorder. During migraine attacks, there is evidence of neuroinflammatory processes characterized by the activation of glial cells, release of pro-inflammatory cytokines, and altered neurotransmitter levels within the trigeminal pain pathway and other brain regions involved in pain processing (24). The release of inflammatory mediators such as interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and calcitonin gene-related peptide (CGRP) can sensitize trigeminal nerve endings, leading to enhanced pain transmission and the characteristic throbbing headache associated with migraines (25). Furthermore, neuroinflammation-induced alterations in synaptic transmission and neuronal excitability may contribute to the hypersensitivity to sensory stimuli (hyperalgesia) and light/sound sensitivity (photophobia and phonophobia) commonly observed during migraine attacks (26). Understanding the role of neuroinflammation in migraines is crucial for developing targeted therapies aimed at modulating these inflammatory processes and alleviating migraine symptoms effectively.
Nutraceuticals and their influence on Neuroinflammation (Appendix 1)
The composition of the final nutraceutical product, Coded as BR3, was developed over a 16-year period in a three-step process. The initial step was to review the science and research behind a group of nutraceutical products that showed a capacity to influence pro-inflammatory pathways, generate mental clarity, and enhance overall mood. The second step was to combine the nutraceuticals into three separate groupings based upon anticipated outcomes: 1. Energy & Clarity, 2. Anti-Inflammatory, and 3. Mood and Energy.
The third step was to test out the products in challenging cases of traumatic brain injury with neuroinflammatory symptomatology.
It has been the mission of the Millennium to understand the causation for debilitating conditions such as neuropsychiatric illnesses and migraine headaches.
Epigallocatechin gallate (EGCG), a major component of green tea, has been shown to reduce neuroinflammation, which is crucial for maintaining cognitive health. EGCG inhibits the production of pro- inflammatory cytokines and reduces the activation of microglia, the immune cells in the brain responsible for inflammation. By modulating these inflammatory pathways, EGCG decreases oxidative stress and protects neurons from damage. This anti-inflammatory action not only helps preserve neuronal health but also enhances cognitive functioning, providing benefits for individuals with neurological conditions such as post-stroke, dementia, and Alzheimer’s disease. (27,28,29,30)
Rhodiola Rosea, an adaptogenic herb, is used to stimulate the nervous system and enhance both physical and mental performance. It helps to treat fatigue, psychological stress, and depression by regulating the release of stress hormones and supporting the body’s ability to adapt to stress. Additionally, Rhodiola rosea boosts energy levels and improves mood by increasing the availability of neurotransmitters like serotonin and dopamine. These combined effects make it a valuable natural remedy for improving overall mental well-being and resilience against stress-related conditions. (31,32,33)
Guarana, a natural stimulant derived from the seeds of the Paullinia cupana plant, is widely used to boost energy levels and enhance cognitive function. It helps to reduce fatigue and improve mental alertness by increasing the release of adrenaline and other stimulating neurotransmitters. Additionally, guarana’s high caffeine content supports concentration and enhances physical performance by stimulating the central nervous system. Its antioxidant properties further protect the brain from oxidative stress, contributing to overall cognitive health and resilience against mental fatigue and stress-related conditions. (34,35,36)
Vitamin B12, (Methyl-Cobalamin) an essential nutrient, plays a crucial role in maintaining nervous system health and enhancing cognitive function. Deficiency expresses itself by a wide variety of hematological, neurological, psychiatric, gastrointestinal, and skin disorders. It supports the production of myelin, the protective sheath around nerves, and aids in the synthesis of neurotransmitters, which are vital for effective communication between nerve cells. Vitamin B12 also helps in the formation of red blood cells, which ensures adequate oxygen delivery to the brain, thereby reducing fatigue and promoting mental clarity. Deficiency in this vitamin can lead to neurological issues, including memory loss and mood disorders. Ongoing research suggested that the imbalance of cytokines and growth factors may be essential to the pathogenesis of the white matter lesions and thus neuropathy due to cobalamin deficiency. By supporting nerve health and cognitive function, Vitamin B12 is essential for overall mental well-being and energy levels. (37,38,39)
Hesperidin, a bioflavonoid found in citrus fruits, offers several health benefits, particularly for the circulatory and nervous systems. It has potent anti-inflammatory and antioxidant properties that help protect neurons from oxidative stress and reduce neuroinflammation. By improving blood flow and strengthening capillaries, hesperidin enhances the delivery of oxygen and nutrients to the brain, which supports cognitive function and overall brain health. Additionally, hesperidin has been shown to have neuroprotective effects, potentially reducing the risk of neurodegenerative diseases and improving mood and mental clarity. These combined benefits make hesperidin a valuable nutrient for maintaining and enhancing cognitive and circulatory health. (40,41,42)
Lepidium meyenii, commonly known as maca root, is a plant native to the high Andes of Peru, renowned for its adaptogenic properties and numerous health benefits. Maca root is used to enhance physical stamina, mental clarity, and overall energy levels. It helps to balance hormones, which can improve mood, reduce symptoms of anxiety and depression, and enhance libido. Additionally, maca root supports cognitive function by increasing blood flow to the brain and providing essential nutrients that support neuronal health.
Its adaptogenic qualities also help the body manage stress more effectively, promoting a sense of well- being and resilience against physical and mental fatigue. (43,44,45)
Docosahexaenoic acid (DHA), an omega-3 fatty acid primarily found in fish oil, is vital for brain health and cognitive function. DHA is a key structural component of brain cell membranes, contributing to their fluidity and function. It plays a crucial role in neuronal growth, development, and communication, which are essential for learning and memory. DHA also has potent anti-inflammatory and antioxidant properties that protect the brain from oxidative stress and reduce neuroinflammation. These combined effects help maintain cognitive function, support mental clarity, and may reduce the risk of neurodegenerative diseases such as Alzheimer’s. A key mechanism of DHA is the protection of neural tissue by the production of Resolvin and Protectin D1 (46,47). In male military personnel, the risk of suicide death was 62% greater with low serum DHA status (48).
Gamma Tocopherol, a form of vitamin E, offers significant health benefits, particularly due to its potent antioxidant and anti-inflammatory properties. It helps protect cells from oxidative damage by neutralizing free radicals, thereby reducing the risk of chronic diseases. In the brain, gamma tocopherol reduces neuroinflammation and supports neuronal health by reducing the production of inflammation by downregulating the production of the transcriptional factor NF-kB, responsible for induction of inflammatory cytokines. Additionally, gamma tocopherol has been shown to enhance immune function and support cardiovascular health by preventing the oxidation of low-density lipoprotein (LDL) cholesterol, which can reduce the risk of atherosclerosis. Overall, gamma tocopherol is a valuable nutrient for maintaining cellular health and protecting against oxidative stress. (49,50,51).
Ascorbic Palmitate – is a highly bioavailable, fat-soluble form of ascorbic acid and possesses all the properties of native water-soluble counterpart, that is vitamin C. It is a potent antioxidant in protecting lipids from peroxidation and is a free radical scavenger reducing oxidative load. Additionally, ascorbate can transfer hydrogen to α-tocopheroxyl radicals and thus regenerate α-tocopherol. Vitamin C also increases the enzyme, glutathione synthetase, that leads to more production of brain and liver glutathione. (52, 53,54)
Quercetin – is a natural polyphenolic flavonoid antioxidant with significant effects on brain metabolism and inflammation reduction. First, quercetin enhances mitochondrial biogenesis by regulating SIRT1 receptors, typically within seven days, resulting in increased ATP (adenosine triphosphate) production. This boost in ATP enhances cellular functions, which can manifest as clearer thoughts, increased energy, and reduced mental fogginess. Second, quercetin downregulates the production of NF-kB, a well-known transcriptional trigger for inflammation, thereby reducing inflammatory responses. Third, quercetin functions as an ionophore, facilitating the transport of zinc into cells, which bolsters antiviral defenses and immune function. Fourth, quercetin upregulates the production of IGF-BP3, an anti-cancer protein induced by healthy levels of growth hormone in the liver, contributing to its protective effects against cancer. (55,56,57,58)
N-Acetyl Cysteine (NAC) is a powerful precursor of glutathione, a critical antioxidant that serves as the brain’s front-line defense against oxidative stress. Following trauma, glutathione levels are often depleted due to increased consumption and damage to the enzymatic systems responsible for its regeneration, leading to an accumulation of harmful free radicals. NAC helps replenish glutathione levels, thereby restoring the brain’s antioxidant capacity and protecting against oxidative damage. Furthermore, NAC inhibits the activation of NF-kB, by suppressing the chemical pathways that trigger its activation. This dual action of replenishing glutathione and inhibiting NF-kB makes NAC a valuable compound for reducing oxidative stress and inflammation in the brain. (59,60,61,62)
Pyrroloquinoline quinone (PQQ) is a compound with diverse and significant health benefits, influencing multiple cellular pathways. It plays a crucial role in neuroprotection and cognitive enhancement by stimulating the production of nerve growth factor (NGF), which supports neuron survival and promotes nerve growth in the brain. This results in improved cognitive performance, including enhanced memoryand attention. PQQ also exhibits potent antioxidant properties, removing free radicals and lowering oxidative stress, thereby protecting cells from damage.
In addition to its neuroprotective effects, PQQ supports mitochondrial health by increasing ATP production and stimulating mitochondrial biogenesis through sirtuin signaling proteins. This boost in mitochondrial function contributes to overall cellular energy and vitality. PQQ also exerts anti-inflammatory effects by downregulating the production of TNF and NF-kB, key inflammatory cytokines, thereby reducing inflammation in the body.
Moreover, PQQ provides cardiovascular protection by improving heart function and reducing the risk of cardiovascular diseases. It enhances muscle health by supporting mitochondrial function in muscle cells, which can lead to improved muscle performance and endurance. (63,64,65,66,67)
Coenzyme Q10 (CoQ10), also known as ubiquinone, is a vital component of the electron transport chain and plays a crucial role in aerobic cellular respiration. This process generates energy in the form of ATP, which powers nearly all cellular activities. Remarkably, 95% of the human body’s energy is produced this way. Organs with the highest energy demands—such as the brain, heart, liver, and kidneys—have the highest concentrations of CoQ10. By facilitating increased ATP production, CoQ10 enhances cellular energy levels, contributing to better and clearer brain function. Additionally, CoQ10’s antioxidant properties protect cells from oxidative damage, further supporting cognitive health and overall well-being. (68,69,70,71,72)
Vitamin B1 (Thiamine) is essential for various aspects of brain health, including neurotransmitter production, memory, mental clarity, cognition, and maintaining a steady gait. It plays a pivotal role in energy metabolism, converting carbohydrates into ATP, the primary energy currency of cells. Thiamine deficiency can lead to conditions like Wernicke-Korsakoff syndrome, characterized by neurological impairments such as confusion and memory loss, which are effectively treated with thiamine supplementation. Beyond its metabolic role, vitamin B1 acts as a potent antioxidant in the brain, protecting neurons from oxidative stress and inflammation. This protective effect helps mitigate neuronal damage caused by free radicals and inflammatory cytokines, supporting overall brain function and cognitive health. (73,74,75)
Vitamin B2, or riboflavin, is indispensable for neurological health and overall well-being. Riboflavin deficiency has been correlated with neurodegenerative disorders and peripheral neuropathy, often presenting symptoms such as cognitive decline and personality changes. Beyond its role in metabolism, riboflavin actively participates in maintaining optimal brain function by exerting anti-inflammatory effects. Specifically, riboflavin acts as a cofactor for enzymes involved in the antioxidant defense system, such as glutathione reductase, which helps neutralize reactive oxygen species (ROS) and mitigate oxidative stress- induced neuroinflammation. Furthermore, riboflavin’s involvement in energy metabolism supports the efficient functioning of neurons, contributing to cognitive processes and overall neurological health. Additionally, vitamin B2’s antioxidant properties may protect against oxidative damage in the brain, further supporting its role in reducing neuroinflammation. (76,77,78,79,80)
Vitamin B5, or pantothenic acid, plays a crucial role as the precursor of coenzyme A (CoA), which is essential for various biological processes. CoA is involved in carbohydrate, lipid, protein, and nucleic acid metabolism, supporting cellular energy production and overall metabolism. Acetyl-CoA, derived from pantothenic acid via CoA, is critical for the synthesis of myelin, the protective sheath around nerves, contributing to neuroprotection. Furthermore, acetyl-CoA is necessary for the production of acetylcholine, a neurotransmitter crucial for brain function, including memory and cognitive processes. Beyond its metabolic roles, pantothenic acid may also exert neuroprotective effects by modulating neuroinflammation and cytokine production, potentially reducing inflammatory responses in the brain. (81,82,83,84)
An open pilot study with active US Marines
I felt like a fortunate soldier in a medical campaign when I was called upon to provide two hours of education at a local Marine base in October 2020. The contacting health officer stated that his commanding officer, a base surgeon, and several officers wanted to learn more about the science behind the work the Millennium had been providing within the military community since 2009. They were aware of the Millennium’s military projects from exposure to different media formats and had been following the work. Upon arrival at the base, I was met with coffee and a healthy breakfast before I was placed in front of a firing line of questions and skepticism. The surgeon made it clear that, after a visit to our website, he was “skeptical” about the work we had been doing for the past 17 years, since there was no published double- blind, cross-over study. The results were based upon an open-study format and therefore, could only be anecdotal without control for placebo effects.
Having been up that road in the past, specifically Portsmouth Naval Hospital Virginia 2018, my retort to the statement of scientific dogma was, 156 medicated, suicidal, non-functional soldiers entered the program and 156 men returned to living, being off medication and ready to face life.
In the lecture, 459 cases were presented with over 78% of the participants achieving a 50% or greater improvement within 12 months (see the latest 2021 Outcome Summary Report at TBIHELPNOW.org/the-science). An array of scientific articles was presented and explained identifying how the Millennium was able to provide a consistent level of recovery for both military and civilian communities. The science focused on two issues; one, the development of an inflammatory environment enveloping the brain, and two, its effect on the molecular biochemistry that regulates cognitive and emotional well-being. At the conclusion of the lecture, questions were taken, and answers were given. At one point the CO stood up and exclaimed that he was excited about the potential of our work based upon the information he was just given. He also stated that I needed to learn how to give a military presentation, to which I accepted. My real reward came when the Surgeon nodded and acknowledged the potential of our program based upon the science.
I left the base and drove back to Encino not knowing what would come next and when. The following day I received an email and had a phone call with the health officer to start the process of setting up a pilot program with 15 – 20 Marines.
The Study Design
Study Title: The Impact of Brain Rescue 3 on Psychological, Physiological, and Physical Functioning in Active-Duty Marines: A 90-Day Pilot Study
Study Objectives:
- Primary Objective:
- To assess the impact of daily administration of Brain Rescue 3 on the psychological, physiological, and physical functioning of active-duty Marines over 90 days.
Study Design:
- Type: Prospective, open-label pilot study
- Duration: 90 days
- Participants: Active-duty Marines (n = 30-50)
Inclusion Criteria:
- Active-duty Marine, aged 18-50
- No major neurological, psychiatric, or medical
- History of symptomatic traumatic brain injury during Study Protocol:
- Screening and Baseline Assessment:
- Participants will complete a 10-page medical history
- Participants will fill out a pre-treatment subjective questionnaire assessing psychological, physiological, and physical functioning. Monthly Program Questionnaire (MPQ).
- Intervention:
- Brain Rescue 3: Consisting of 1 teaspoon each of Clear Mind & Energy, Brain Care 2, and B is for Brain, taken prior to breakfast for the entire 90-day period.
- Assessment Schedule:
- Day 0 (Baseline): Complete the pre-treatment subjective
- Day 30: Complete the Monthly Program Questionnaire (MPQ) evaluating 18 points of
- Day 60: Complete the
- Day 90: Complete the
- Outcome Measures:
- Primary Outcome: Changes in subjective assessments of psychological, physiological, and physical functioning as measured by the MPQ at 30, 60, and 90 days.
- Secondary Outcome: Adherence to the supplementation regimen and any reported adverse
- Data Analysis:
- Individual and group results will be plotted to assess trends over
- Statistical analysis will be conducted to compare baseline and follow-up scores using appropriate methods.
Expected Outcomes:
- Improvement in psychological, physiological, and physical functioning is anticipated, providing preliminary evidence for the efficacy of Brain Rescue 3 in this population.
Results from the Monthly Program Questionnaires
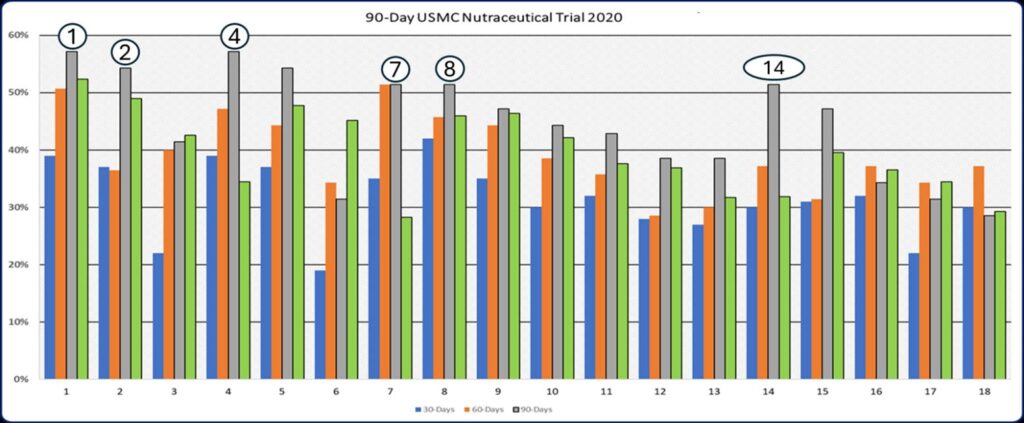
Outcome Graph 1: Participant were started on the Tri-Pak Protocol (BR3) and asked to rate 18 key factors relative to psychological, physical, and physiological functioning after 30, 60 and 90-days. Seven areas; increased mental energy, improved sleep, improved emotional stability, improved memory, increased sense of well-being, reduced anxiety, and reduced joint aches and pain had peak improvements during the 90 days of their program. Additionally, migraines improved by an average of 28%, libido by 43%, more physical energy by 45%, and improved strength during exercise by 42%. The data points derivedd from the monthly program questionnaires (MPQ) were used to construct this graph
I have noticed an increase in mental energy.
- My sleep has
- I am sleeping less and waking up feeling more
- My overall emotional state has
- My overall memory has
- My libido (sex drive) has
- I have an increased sense of well-
- I feel calmer under stress, less
- I have generally more physical energy to do more
- When I exercise, I have more energy and feel
- I can perform physically longer without the expected
- My athletic performance has improved
- I recover faster after
- Joint Aches and pains are
- Facial texture has
- Wrinkles have decreased
- Skin thickness has increased. 18: My Migraines have
Reviewing the Results
Eighteen non-standardized, subjective markers were employed to evaluate the progress of each Marine participating in a daily regimen of a pre-measured nutraceutical product. The Monthly Program Questionnaire (MPQ) was administered every 30 days, with results compiled and analyzed across the entire cohort. As illustrated in Outcome Graph 1, there were significant improvements in markers related to mental energy [1], sleep quality [2], emotional stability [4], sense of well-being [7], reduced anxiety [8], and a decrease in migraines [18] within this randomized, prospective, anecdotal study.
Overall, these findings suggest a broad spectrum of improvements, which we attribute to the resolution or reduction of inflammation, as depicted in Chart 1.
Chart 1: Conditions associated with Neuroinflammation
| Classification | Symptom | Neuroinflammation Impact | Reference Article |
|
Mood Disorders |
Depression |
Neuroinflammation increases pro- inflammatory cytokines, affecting serotonin metabolism. | Felger, J. C. (2017). Role of Inflammation in Depression and Treatment Implications. Handbook of Experimental Pharmacology. |
|
Anxiety |
Inflammatory cytokines disrupt neurotransmitter signaling. |
Miller, A. H., & Raison, C. L. (2016). The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nature Reviews Immunology. | |
| Neurodegenerative Conditions | Alzheimer’s Disease | Chronic neuroinflammation contributes to amyloid plaque formation and tau hyperphosphorylation. | Heneka, M. T., et al. (2015). Neuroinflammation in Alzheimer’s Disease. The Lancet Neurology. |
| Multiple Sclerosis | Inflammation leads to demyelination and neuronal damage. | Lassmann, H., et al. (2015). The Immunopathology of Multiple Sclerosis: An Overview. Brain Pathology. | |
| Parkinson’s Disease | Microglial activation and neuroinflammatory cytokines contribute to dopaminergic neuron death. | Collins, L. M., et al. (2016). Microglia in Alzheimer’s Disease and Parkinson’s Disease: A Role for Neuroinflammation. Molecular Neurobiology. | |
|
Cognitive Issues |
Memory Impairment |
Cytokine-induced neuroinflammation disrupts hippocampal function. |
Yirmiya, R., & Goshen, I. (2017). Immune Modulation of Learning, Memory, Neural Plasticity, and Neurogenesis. Brain, Behavior, and Immunity. |
| Impaired Executive Function | Inflammation affects prefrontal cortex activity. Considered to be due to loss of key neurosteroids: DHEA-s, pregnenolone-s, and allopregnanolone-s | Marsland, A. L., et al. (2015). The Effects of Acute Psychological Stress on Circulating and Stimulated Inflammatory Markers: A Meta- Analysis and Systematic Review. Brain, Behavior, and Immunity. | |
| Sleep Disorders | Insomnia | Elevated levels of pro-inflammatory cytokines disrupt sleep homeostasis. | Irwin, M. R., et al. (2016). Inflammation and Sleep: From Stress to Sleeplessness. Nature Reviews Neuroscience. |
|
Migraines |
Chronic Migraines |
Inflammatory cytokines sensitize trigeminal nerve pathways. |
Vecsei, L., et al. (2017). Migraine, Neurogenic Inflammation, Drug Development – Pharmacology and Therapeutics. Frontiers in Pharmacology. |
|
Tinnitus |
Persistent Tinnitus | Neuroinflammation affects auditory pathways, contributing to tinnitus perception. | Shore, S. E., et al. (2016). Mechanisms of Noise- Induced Tinnitus: Insights from Cellular Studies. Hearing Research. |
| Neuromuscular Illnesses | Myasthenia Gravis | Autoimmune neuroinflammation affects acetylcholine receptors at the neuromuscular junction. | Meriggioli, M. N., & Sanders, D. B. (2016). Autoimmune Myasthenia Gravis: Emerging Clinical and Biological Heterogeneity. The Lancet Neurology. |
| Hyperalgesia | Pain Sensitization | Neuroinflammation contributes to central and peripheral sensitization. Peroxynitrite. | Ji, R. R., et al. (2016). Emerging Roles of Immune Cells in Chronic Pain. Nature Medicine. |
|
Other Issues |
Fatigue |
Inflammatory cytokines induce central fatigue via the hypothalamus. |
Dantzer, R., et al. (2018). From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nature Reviews Neuroscience. |
Discussion
The activation of pro-inflammatory pathways following various traumatic events is a fundamental process in the development of neuropsychiatric disorders and physical pain syndromes (85). As the brain’s microenvironment becomes increasingly saturated with pro-inflammatory cytokines and reactive oxygen species (ROS), critical biochemical processes, including neurotransmitter synthesis, enzymatic functions, and the neutralization of toxic metabolic by-products, begin to deteriorate (86). Over time, disruptions in these pathways, particularly those involving neurosteroids and neuroactive steroids, compromise neuroprotection, leading to neuronal and glial cell death (87). The extent of cellular loss correlates directly with the disruption of neural circuits that regulate cognition, motivation, and emotional stability.
Thus, the primary therapeutic objective is to resolve the inflammatory environment to facilitate the restoration of essential neurochemical pathways. Brain Rescue 3 (BR3), comprising 16 nutraceuticals, is specifically designed to mitigate key biological triggers of inflammation, as detailed in the section “Nutraceuticals and Their Influence on Neuroinflammation.”
Primary trauma, whether physical or psychological, and whether involving subconcussive or concussive forces, induces cellular damage and triggers secondary trauma cascades (88). Depending on the nature of the trauma, cellular integrity may be compromised, leading to the leakage of intracellular components into the extracellular space (89). This process often affects the mitochondria, resulting in the excessive production of ROS as they attempt to generate additional ATP (adenosine triphosphate) to meet the heightened energy demands imposed by trauma (90). During cellular repair, ATP is required in substantial amounts. However, ROS, as free radicals, promote the formation of reactive nitrogen species (RNS), such as peroxynitrite (PN). Peroxynitrite has been shown to impair enzyme systems essential for synthesizing serotonin, melatonin, and glutathione, thereby increasing the risk of depression, insomnia, and Parkinson’s disease, respectively (91).
When a singular, minor injury occurs, secondary trauma-induced damage can be self-limiting, presenting transient symptoms akin to those seen in viral infections, including fatigue, headache, myalgia, emotional lability, and anorexia. However, in cases of repetitive head injuries, whether subconcussive or concussive, secondary trauma can become chronic. Under these conditions, the continuous production of oxidative stress and pro-inflammatory cytokines exacerbates neurodegenerative processes. If left unchecked, this progressive damage accumulates, resulting in symptoms that correspond to the degree and extent of injury across different brain regions. Concurrently, neurochemical dysregulation disrupts biochemical processes vital for maintaining healthy brain function. Ultimately, individuals may be misdiagnosed with psychiatric disorders and subjected to polypharmacy.
In this 90-day open-label study, 18 Marines awaiting medical discharge were administered a morning regimen of Brain Rescue 3, a comprehensive nutraceutical formulation developed to reduce free radicals and attenuate the production and release of pro-inflammatory cytokines. Utilizing the Millennium Program Questionnaire (MPQ), a subjective self-assessment tool, 65% of participants reported a 50% to 100% improvement in symptoms by the end of the study period. The remaining 35% who did not achieve at least a 50% improvement were found to have one or more lifestyle factors (Appendix 2) that either blocked or impeded the reduction of pro-inflammatory cascades.
Conclusion
Between the onset of Operation Enduring Freedom (OEF) in 2001 and the withdrawal from Afghanistan in August 2021, a significant number of veterans have been diagnosed with psychiatric conditions. The most common diagnoses include Post-Traumatic Stress Disorder (PTSD), depression, anxiety disorders, and substance use disorders. It is estimated that over 1.7 million veterans from the OEF, Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) conflicts have received care through the Veterans Health Administration (VHA). Among these veterans, approximately 29% (493,000) have been diagnosed with at least one mental health condition, with PTSD being one of the most prevalent diagnoses.
These high rates of psychiatric diagnoses are likely due to the intense and prolonged nature of combat exposure in these conflicts, which involved constant threats such as roadside bombs and improvised explosive devices. Additionally, factors such as multiple deployments and the unique stressors faced by female service members, including higher exposure to combat and sexual trauma, have contributed to these elevated rates of mental health issues among veterans.
The data highlights the critical need for continued support and mental health care for veterans who served in these conflicts (Psychiatric Times).
From 2001 to 2021, the U.S. Department of Defense recorded over 450,000 cases of traumatic brain injuries (TBIs) among service members. This includes both concussive and subconcussive TBIs, which were prevalent among soldiers deployed in combat zones. The peak year was 2011, with nearly 33,000 reported cases, and mild TBIs, often resulting from concussive events like explosions, accounted for the majority of these injuries (Military Health, Brain Injury Association of MI )
Nearly one million soldiers, that we know about, are suffering with the consequences of neuroinflammation either labeled as traumatic brain injury or the most common psychiatric label, PTSD. Nonetheless, it my operational opinion that both forms of presentation are from the consistent underlying neuroinflammation which causes disruption of the brain’s neurochemistry.
Appendix 1: Brain Rescue 3 – Nutraceuticals, Neuroinflammation and Benefits to Brain Chemistry
| Compound | Benefits Related to Neuroinflammation | Beneficial Effects on Cytokines | Influence on Brain Chemistry | References |
|
Epigallocatechin Gallate (EGCG) |
– Reduces neuroinflammation by inhibiting microglial activation. – Decreases oxidative stress, protecting neurons from damage. |
– Inhibits production of pro- inflammatory cytokines such as TNF-α and IL-6. – Modulates inflammatory pathways to reduce overall cytokine levels. |
– Enhances cognitive functioning.
– Protects against neuronal damage, supporting conditions like Alzheimer’s disease. – Promotes neuronal health and cognitive clarity. – Decreases Cortisol. |
(1c, 2c, 3c, 4c) |
|
Rhodiola Rosea |
– Enhances resilience against stress-induced neuroinflammation. – Supports nervous system health by regulating stress responses. |
– Regulates release of stress hormones, indirectly modulating cytokine production. – Reduces levels of cortisol, which can influence inflammatory cytokines. |
– Increases availability of neurotransmitters like serotonin and dopamine.
– Boosts mental performance and reduces symptoms of fatigue and depression. – Enhances mood and cognitive function via neurotransmitters. – Decreases Cortisol. |
(5c, 6c, 7c) |
|
Guarana |
– Provides antioxidant protection, reducing oxidative stress-related neuroinflammation. – Enhances cognitive resilience against mental fatigue. |
– May indirectly influence cytokine levels by reducing oxidative stress. – Supports immune function through its antioxidant properties. |
– Increases release of adrenaline and stimulating neurotransmitters.
– Enhances mental alertness and concentration. – Boosts physical performance by stimulating the CNS. |
(8c, 9c, 10c) |
|
Vitamin B12 (Methyl- Cobalamin) |
– Supports myelin sheath integrity, providing neuroprotection. – Reduces neuroinflammation by maintaining healthy nerve function. |
– Helps balance cytokines and growth factors, potentially reducing inflammatory responses associated with neuropathy. – May influence cytokine production related to neurological health. |
– Essential for neurotransmitter synthesis, enhancing neuronal communications.
– Promotes cognitive function and mental clarity. – Ensures adequate oxygen delivery to the brain, reducing fatigue and increases cognition. |
(11c, 12c, 13c) |
|
Hesperidin |
– Reduces neuroinflammation through its anti-inflammatory properties. – Protects neurons from oxidative stress. |
– Decreases production of inflammatory cytokines. – Modulates immune responses to support reduced inflammation in the nervous system. |
– Enhances blood flow and nutrient delivery to the brain.
– Supports cognitive function and mental clarity. – May reduce the risk of neurodegenerative diseases. |
(14, 15, 16) |
|
Lepidium Meyenii (Maca Root) |
– Supports neuroprotection by enhancing neuronal health. – Helps the body manage stress, reducing stress-induced neuroinflammation. |
– Improves hormonal balance disrupted by pro-inflammatory cytokines. – Reduces anxiety and depression caused by pro-inflammatory cytokine hormonal disruption. |
– Increases blood flow to the brain, supporting cognitive function.
– Enhances mental clarity and energy levels. – Balances neurotransmitters, improving mood and reducing mental fatigue. – Decreases Cortisol |
(17c, 18c, 19c) |
|
Docosahexaenoic Acid (DHA) |
– Protects neural tissue by reducing neuroinflammation. – Decreases oxidative stress through antioxidant activity. |
– Downregulates pro- inflammatory cytokines like TNF-α and IL-1β. – Promotes the production of anti- inflammatory molecules like Resolvin and Protectin D1. |
– Essential for brain cell membrane function.
– Supports neuronal growth and communication, enhancing learning and memory. – Reduces neurodegenerative diseases by maintaining cognitive health. – increases Survivin & Protectin. |
(20c, 21c, 22c) |
|
Gamma Tocopherol (Vitamin E) |
– Reduces neuroinflammation by downregulating NF-κB. – Protects neurons from oxidative damage. |
– Lowers production of inflammatory cytokines by inhibiting NF-κB.
– Enhances immune function, supporting reduced inflammatory responses. |
– Protects cells from oxidative stress.
– Supports cardiovascular health. – Maintains cellular integrity and supports overall cognitive function. |
(23c, 24c, 25c) |
| Compound | Benefits Related to Neuroinflammation | Beneficial Effects on Cytokines | Influence on Brain Chemistry | References |
|
Ascorbic Palmitate (Vitamin C) |
– Protects lipids from peroxidation, reducing oxidative stress-related neuroinflammation. – Acts as a free radical scavenger in the brain. |
– Reduces oxidative load, which can influence cytokine production. – Enhances glutathione synthesis, supporting the antioxidant defense system. |
– Regenerates α-tocopherol (Vitamin E), maintaining antioxidant protection.
– Increases glutathione in the brain, enhancing antioxidant. – Improved antioxidant defenses and free radical scavenging. |
(26, 27, 28) |
|
Quercetin |
– Downregulates NF-κB, reducing neuroinflammation. – Enhances mitochondrial biogenesis, supporting neuronal energy and health. |
– Reduces production of pro- inflammatory cytokines. – Upregulates IGF-BP3, contributing to anti-cancer and anti-inflammatory effects. |
– Enhances ATP production, improving cellular energy and cognitive function.
– Acts as an ionophore for zinc, boosting immune function. – Supports clearer thoughts and reduces mental fogginess through improved mitochondrial function. |
(29, 30, 31, 32) |
|
N-Acetyl Cysteine (NAC) |
– Replenishes glutathione levels, reducing oxidative stress-induced neuroinflammation. – Inhibits NF-κB activation, decreasing inflammatory responses. |
– Suppresses pathways that activate NF-κB, leading to lower pro-inflammatory cytokine levels. – Restores antioxidant defenses, indirectly modulating cytokine production. |
– Restores brain’s antioxidant capacity, protecting against oxidative damage.
– Supports neurotransmitter balance and cognitive function. – Reduces inflammation, promoting resilience against neurological stress. |
(33, 34, 35, 36) |
|
Pyrroloquinoline Quinone (PQQ) |
– Reduces inflammation by decreasing TNF-α and NF-κB. – Enhances neuroprotection through nerve growth factor (NGF) stimulation. |
– Lowers pro-inflammatory cytokines such as TNF-α. – Modulates immune responses to reduce systemic and neural inflammation. |
– Stimulates mitochondrial biogenesis, increasing ATP production and cellular energy.
– Supports cognitive functions like memory and attention. – Promotes neuronal survival and growth. |
(37, 38, 39, 40, 41) |
|
Coenzyme Q10 (CoQ10) |
– Protects neurons from oxidative stress, reducing neuroinflammation. – Enhances mitochondrial function, supporting neuronal energy and health. |
– Its antioxidant properties may indirectly reduce pro- inflammatory cytokines.
– Supports cellular energy (ATP), which modulates immune responses and cytokine production. |
– Facilitates ATP production enhancing cellular energy. – Protects cells from oxidative damage, supporting cognition. – Maintains mitochondrial integrity. |
(42, 43, 44, 45, 46) |
|
Vitamin B1 (Thiamine) |
– Acts as an antioxidant, protecting neurons from oxidative stress- induced inflammation. – Mitigates neuronal damage caused by inflammatory cytokines. |
– Reduces production of inflammatory cytokines through its antioxidant activity. – Supports the body’s defense against oxidative stress, indirectly modulating cytokine levels. |
– Essential for neurotransmitter production, memory, and cognitive clarity.
– Enhances energy metabolism by increasing ATP production. – Supports overall brain function and cognitive health through energy production and antioxidant protection. |
(47, 48, 49) |
|
Vitamin B2 (Riboflavin) |
– Acts as a cofactor for antioxidant enzymes, reducing oxidative stress-induced neuroinflammation. – Supports efficient neuronal function, contributing to neuroprotection. |
– Neutralizes reactive oxygen species (ROS) via glutathione reductase, mitigating cytokine- induced inflammation.
– Enhances the antioxidant defense system, reducing pro- inflammatory cytokines. |
– Improved energy metabolism.
– Protects against oxidative damage in the brain, enhancing overall neurological health. – Maintains efficient neuronal functioning through energy support and antioxidant activity. |
(50, 51, 52, 53, 54) |
|
Vitamin B5 (Pantothenic Acid) |
– Modulates neuroinflammation by regulating cytokine production. – Supports myelin synthesis, providing neuroprotection. |
– Reduces inflammatory responses in the brain by modulating cytokine production. – Supports the body’s ability to manage inflammation through CoA-related metabolic pathways. |
– Precursor to acetyl-CoA, essential for synthesis of acetylcholine.
– Enhances energy metabolism, supporting cognitive function. – Facilitates synthesis of myelin, protecting nerve cells and promoting efficient neural communication. – Decreases Cortisol. |
(55, 56, 57, 58) |
Appendix 2: Lifestyle issues affecting neuroinflammation.
| Lifestyle Issue | Mechanism | Impact on Neuroinflammation |
|
Alcohol Consumption |
Ethanol metabolism produces acetaldehyde and ROS, which cause oxidative stress and damage to neurons. |
Increases neuroinflammation by upregulating pro- inflammatory cytokines like TNF-α and IL-6. |
| Eating Inflammatory Foods | High intake of trans fats, refined sugars, and processed foods trigger systemic inflammation. | Promotes neuroinflammation by increasing the permeability of the blood-brain barrier and activating microglia, upregulating pro-inflammatory cytokines. |
|
Poor Hydration |
Dehydration leads to reduced blood flow and impaired nutrient delivery to the brain. | Worsens neuroinflammation by increasing oxidative stress and impairing the brain’s detoxification processes. Activation of microglia with the production of pro-inflammatory cytokines. |
|
Poor Nutrition |
Deficiency in essential nutrients (e.g., omega-3s, vitamins, antioxidants) impairs brain function. |
Amplifies neuroinflammation by failing to counteract oxidative stress and inflammation. |
|
Poor Exercise |
Sedentary behavior reduces neurogenesis and increases oxidative stress. |
Contributes to neuroinflammation by lowering anti- inflammatory cytokines and increasing ROS production. |
|
Poor Sleep Hygiene |
Disrupted sleep patterns impair the glymphatic system, which clears toxins from the brain. |
Heightens neuroinflammation by allowing the accumulation of amyloid-beta and other inflammatory agents. |
| Dysbiosis and Poor Gut Health | Imbalance in gut microbiota produces endotoxins and disrupts the gut-brain axis. |
Increases neuroinflammation by allowing the translocation of pro-inflammatory molecules across the gut lining. |
| Medication (Gabapentin, Pregabalin, Narcotics) | These medications may alter neurotransmitter levels and impair mitochondrial function. |
Can exacerbate neuroinflammation by increasing oxidative stress and disrupting normal brain signaling. |
Additional Reading
**Standardized Test vs Non-standardized Test (Neurocognitive Assessment Tips)
References for Section A: Introduction
- Wohleb ES, Franklin T, Iwata M, et Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17(8):497-511.
- Uttara, , Singh, A. V, Zamboni, P., & Mahajan, R. T. (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology, 7(1), 65–74. https://doi.org/10.2174/157015909787602823
- Farooqui, A., Horrocks, L. A., & Farooqui, T. (2007). Modulation of inflammation in brain: A matter of fat. Journal of Neurochemistry, 101(3), 577–599. https://doi.org/10.1111/j.1471-4159.2006.04371.x
- Lochhead, J., Yang, J., Ronaldson, P. T., & Davis, T. P. (2020). Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Frontiers in Physiology, 11(August). https://doi.org/10.3389/fphys.2020.00914
- Corps, N., Roth, T. L., & McGavern, D. B. (2015). Inflammation and neuroprotection in traumatic brain injury. JAMA Neurology, 72(3), 355–362. https://doi.org/10.1001/jamaneurol.2014.3558
- Rego, J. Do, Seong, J. Y., Burel, D., Leprince, J., Vaudry, D., Luu-The, V., Tonon, M. C., Tsutsui, K., Pelletier, G., & Vaudry, H. (2012). Regulation of neurosteroid biosynthesis by neurotransmitters and neuropeptides. Frontiers in Endocrinology, 3(JAN), 1–15. https://doi.org/10.3389/fendo.2012.00004
- Wu, , & Zhang, J. (2023). Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis. Journal of Neuroinflammation, 20(1), 1–20. https://doi.org/10.1186/s12974-023-02964-x
- Gordon, (2023). Neuroinflammation: The Road to Neuropsychiatric Illnesses. www.tbihelpnow.org/the-science
- Mendes Arent, , Souza, L. F. De, Walz, R., & Dafre, A. L. (2014). Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. BioMed Research International, 2014. https://doi.org/10.1155/2014/723060
- Gan, S., Stein, S. C., Swanson, R., Guan, S., Garcia, L., Mehta, D., & Smith, D. H. (2019). Blood biomarkers for traumatic brain injury: A quantitative assessment of diagnostic and prognostic accuracy. Frontiers in Neurology, 10(APR). https://doi.org/10.3389/fneur.2019.00446
- Rao, , Syeda, A., Roy, D., Peters, M. E., & Vaishnavi, S. (2017). Neuropsychiatric aspects of concussion: acute and chronic sequelae. Concussion, 2(1), CNC29. https://doi.org/10.2217/cnc-2016-0018
- Hernandez-Ontiveros, G., Tajiri, N., Acosta, S., Giunta, B., Tan, J., & Borlongan, C. V. (2013). Microglia Activation as a Biomarker for Traumatic Brain Injury. Frontiers in Neurology, 4(March), 1–9. https://doi.org/10.3389/fneur.2013.00030
- Kölliker-Frers, R., Udovin, L., Otero-Losada, M., Kobiec, T., Herrera, M. I., Palacios, J., Razzitte, G., & Capani, F. (2021). Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Mediators of Inflammation, 2021. https://doi.org/10.1155/2021/9999146
- Réus, Z., Fries, G. R., Stertz, L., Badawy, M., Passos, I. C., Barichello, T., Kapczinski, F., & Quevedo, J. (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience, 300, 141–154. https://doi.org/10.1016/j.neuroscience.2015.05.018
- Bauer, E., & Teixeira, A. L. (2019). Inflammation in psychiatric disorders: What comes first? Annals of the New York Academy of Sciences, 1437(1), 57–67. https://doi.org/10.1111/nyas.13712
- Rhie, J., Jung, E. Y., & Shim, I. (2020). The role of neuroinflammation on pathogenesis of affective disorders. Journal of Exercise Rehabilitation, 16(1), 2–9. https://doi.org/10.12965/jer.2040016.008
- Olmos, , & Lladó, J. (2014). Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediators of Inflammation, 2014. https://doi.org/10.1155/2014/861231
- Zheng, , & Tong, W. (2015). Understanding the neurotransmitter changes underlying cognitive dysfunction in traumatic brain injury and possible therapeutic targets: A review. Archives of Medical Science, 11(3), 696–698. https://doi.org/10.5114/aoms.2015.52380
- Tian, , Hui, C. W., Bisht, K., Tan, Y., Sharma, K., Chen, S., Zhang, X., & Tremblay, M. E. (2017). Microglia under psychosocial stressors along the aging trajectory: Consequences on neuronal circuits, behavior, and brain diseases. Progress in Neuro- Psychopharmacology and Biological Psychiatry, 79(January), 27–39. https://doi.org/10.1016/j.pnpbp.2017.01.007
- Bauer, E., & Teixeira, A. L. (2021). Neuroinflammation in Mood Disorders: Role of Regulatory Immune Cells.
NeuroImmunoModulation. https://doi.org/10.1159/000515594
- Vythilingam, , Vermetten, E., Anderson, G. M., Luckenbaugh, D., Anderson, E. R., Snow, J., Staib, L. H., Charney, D. S., & Bremner, J.
- (2004). Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biological Psychiatry, 56(2), 101–112. https://doi.org/10.1016/j.biopsych.2004.04.002
- Kaplan, B., Leite-Morris, K. A., Wang, L., Rumbika, K. K., Heinrichs, S. C., Zeng, X., Wu, L., Arena, D. T., & Teng, Y. D. (2017). Pathophysiological Bases of Comorbidity: Tbi and Ptsd. Journal of Neurotrauma, October, neu.2016.4953. https://doi.org/10.1089/neu.2016.4953
- Milior, G., Lecours, C., Samson, L., Bisht, K., Poggini, S., Pagani, F., Deflorio, C., Lauro, C., Alboni, S., Limatola, C., Branchi, I., Tremblay, -E., & Maggi, L. (2016). Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain, Behavior, and Immunity, 55, 114–125. https://doi.org/10.1016/j.bbi.2015.07.024
- Manuscript, , Root, D., Produces, C., Axonal, M., & Hypersensitivity, B. (2009). Cytokines, Inflammation and Pain. Int Anesthesiol Clin., 69(2), 482–489. https://doi.org/10.1097/AIA.0b013e318034194e.Cytokines
- Manuscript, , Root, D., Produces, C., Axonal, M., & Hypersensitivity, B. (2009). Cytokines, Inflammation and Pain. Int Anesthesiol Clin., 69(2), 482–489. https://doi.org/10.1097/AIA.0b013e318034194e.Cytokines25
- Brandt, B., Doesborg, P. G. G., Haan, J., Ferrari, M. D., & Fronczek, R. (2020). Pharmacotherapy for Cluster Headache. CNS Drugs, 34(2), 171–184. https://doi.org/10.1007/s40263-019-00696-2
- Menard, , Bastianetto, S., & Quirion, R. (2013). Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Frontiers in Cellular Neuroscience, 7(December), 1–8. https://doi.org/10.3389/fncel.2013.00281
- Zhang, , Wang, B., Cao, S., & Wang, Y. (2015). Epigallocatechin-3-gallate (EGCG) attenuates traumatic brain injury by inhibition of edema formation and oxidative stress. Korean Journal of Physiology and Pharmacology, 19(6), 491–497. https://doi.org/10.4196/kjpp.2015.19.6.491
- Kelsey, A., Wilkins, H. M., & Linseman, D. A. (2010). Nutraceutical Antioxidants as Novel Neuroprotective Agents. Molecules, 15(11), 7792–7814. https://doi.org/10.3390/molecules15117792
- Menard, , Bastianetto, S., & Quirion, R. (2013). Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Frontiers in Cellular Neuroscience, 7(December), 1–8. https://doi.org/10.3389/fncel.2013.00281
- Pu, W. ling, Zhang, Ying, Bai, R. Yu, Sun, L. Kang, Li, W. Hua, Yu, Y. li, Zhang, Y., Song, L., Wang, Z. Xin, Peng, Y. Fei, Shi, H., Zhou, K., & Li, T. Xiang. (2020). Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomedicine and Pharmacotherapy, 121(October 2019). https://doi.org/10.1016/j.biopha.2019.109552
- Jacob, , Nalini, G., & Chidambaranathan, N. (2013). Neuroprotective effect of Rhodiola rosea linn against MPTP induced cognitive impairment and oxidative stress. Annals of Neurosciences, 20(2), 47–51. https://doi.org/10.5214/ans.0972.7531.200204
- Eroglu , Toprak S., Urgan O, MD, Ozge E. Onur, MD, Arzu Denizbasi, MD, Haldun Akoglu, MD, Cigdem Ozpolat, MD, Ebru Akoglu,
- (2012). Rhodiola rosea A Phytomedicinal Overview. Saudi Med J, 33, 3–8. https://doi.org/10.1073/pnas.0703993104
- Konstantinos, , & Heun, R. (2019). The effects of Guarana (Paullinia cupana) supplementation on the cognitive performance of young healthy adults – a Systematic Review. Global Psychiatry, 2(2), 171–182. https://doi.org/10.2478/gp-2019-0015
- Yamaguchi, K. K. de L., & Souza, A. de O. (2020). Antioxidant, Hypoglycemic and Neuroprotective Activities of Extracts from Fruits Native to the Amazon Region: A Biotechnology Journal International, 24(6), 9–31. https://doi.org/10.9734/bji/2020/v24i630119
- Hui, H., Tang, G., & Go, V. L. W. (2009). Hypoglycemic herbs and their action mechanisms. Chinese Medicine, 4, 1–11. https://doi.org/10.1186/1749-8546-4-11Fangfang Wu1, Ke Xu, (2019).Vitamin B12 enhances nerve repair and improves functional recovery after traumatic brain injury by inhibiting ER stress-induced neuron injury. Frontiers in Pharmacology
- Kennedy, O. (2016). B Vitamins and the Brain: Mechanisms, Dose and Efficacy–A Review. Nutrients, 8(2), 68. https://doi.org/10.3390/nu8020068
- Selhub, , Troen, A., & Rosenberg, I. H. (2010). B vitamins and the aging brain. Nutrition Reviews, 68(SUPPL. 2). https://doi.org/10.1111/j.1753-4887.2010.00346.x
- Hajialyani, , Farzaei, M. H., Echeverría, J., Nabavi, S. M., Uriarte, E., & Eduardo, S. S. (2019). Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules, 24(3). https://doi.org/10.3390/molecules24030648
- Lee, K., Hyun, S. W., & Jung, Y. S. (2020). Yuzu and hesperidin ameliorate blood-brain barrier disruption during hypoxia via antioxidant activity. Antioxidants, 9(9), 1–15. https://doi.org/10.3390/antiox9090843
- Kim, J., Wie, B., Ahn, M., Tanaka, A., Matsuda, H., & Shin, T. (2019). Benefits of hesperidin in central nervous system disorders: A review. Anatomy and Cell Biology, 52(4), 369–377. https://doi.org/10.5115/acb.19.119
- Guo, S. S., Gao, X. F., Gu, R., Wan, Z. X., Lu, A. M., Qin, Z. H., & Luo, L. (2016). Preservation of Cognitive Function by Lepidium meyenii (Maca) Is Associated with Improvement of Mitochondrial Activity and Upregulation of Autophagy-Related Proteins in Middle- Aged Mouse Cortex. Evidence-Based Complementary and Alternative Medicine, 2016. https://doi.org/10.1155/2016/4394261
- Pino-Figueroa, , Nguyen, D., & Maher, T. J. (2010). Neuroprotective effects of Lepidium meyenii (Maca): Annals of the New York academy of sciences. Annals of the New York Academy of Sciences, 1199, 77–85. https://doi.org/10.1111/j.1749-6632.2009.05174.x
- Korkmaz, (2018). Antioxidants in Maca (Lepidium meyenii) as a Supplement in Nutrition. Antioxidants in Foods and Its Applications. https://doi.org/10.5772/intechopen.75582
- Richer, C. (2017). Functional Medicine Approach to Traumatic Brain Injury. Medical Acupuncture, 29(4), 206–214. https://doi.org/10.1089/acu.2017.1217
- Erdman J, Oria M, Pillsbury L, (2011). Nutrition and Traumatic Brain Injury: Improving Acute and Subacute Health Out- comes in Military Personnel. Washington, DC: The National Academies Press; 2011. 13121.pdf (deploymentpsych.org)
- Lewis, D. (2012). Suicide Deaths of Active Duty U.S. Military and Omega-3 Fatty Acid Status: A Case Control Comparison. J Clin Psychiatry, 72(12), 1585–1590. https://doi.org/10.4088/JCP.11m06879.Suicide
- Son, , Le, N. T., Pannacciulli, N., Chen, K., Salbe, A. D., Hill, J. O., Rena, R., Reiman, E. M., & Krakoff, J. (2007). Tocotrienols: Vitamin E Beyond Tocopherols Chandan. 86(3), 573–579. https://doi.org/10.1109/TMI.2012.2196707.Separate
- Mason, (2009). Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. 19(6), 389–399. https://doi.org/10.1016/j.asieco.2008.09.006.EAST
- Milatovic, , Zaja-Milatovic, S., Montine, K. S., Shie, F. S., & Montine, T. J. (2004). Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immune response in vivo. Journal of Neuroinflammation, 1, 1–7. https://doi.org/10.1186/1742-2094-1-20
- Cha, J., Kim, H., Kim, Y., & Kim, (2011). Anti-inflammatory effect of ascorbyl palmitate in the brain. Journal of Medicinal Food, 14(4), 332-339. DOI: 10.1089/jmf.2010.0061.
- Li, , Horke, S., Förstermann, U. (2014). Vascular oxidative stress, nitric oxide and inflammation in atherosclerosis. Journal of Molecular Medicine, 92, 1053-1063. DOI: 10.1007/s00109-014-1189-9
- Cervantes Gracia, , Llanas-Cornejo, D., & Husi, H. (2017). CVD and Oxidative Stress. Journal of Clinical Medicine, 6(2), 22. DOI: 10.3390/jcm6020022.
- Boots, W., Haenen, G.R., & Bast, A. (2008). Health effects of quercetin: From antioxidant to nutraceutical. European Journal of Pharmacology, 585(2-3), 325-337.
- Russo, , Spagnuolo, C., Tedesco, I., & Russo, G.L. (2012). The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochemical Pharmacology, 83(1), 6-15.
- Dabbagh-Bazarbachi, , Clergeaud, G., Quesada, I.M., Ortiz, M., & Garcia, M.L. (2014). Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses, 6(6), 2467-2487.
- Spencer, P. (2008). Flavonoids: modulators of brain function? British Journal of Nutrition, 99 E Suppl 1, ES60-77.
- McCarty, F., & DiNicolantonio, J.J. (2015). Neuroprotective Effects of N-Acetylcysteine and Glutathione. Medical Hypotheses, 85(1), 59-62.
- Aldini, , Altomare, A., Baron, G., Vistoli, G., Carini, M., Borsani, L., & Sergio, F. (2018). N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why. Free Radical Research, 52(7), 751-762.
- Samuni, Y., Goldstein, S., Dean, O.M., & Berk, (2013). The Chemistry and Biological Activities of N-Acetylcysteine. Biochimica et Biophysica Acta (BBA) – General Subjects, 1830(8), 4117-4129.
- De Flora, , D’Agostini, F., Balansky, R., Micale, R.T., & La Maestra, S. (2016). Antioxidant Activity and Other Mechanisms of Thiols Involved in Chemoprevention of Mutation and Cancer. The American Journal of Clinical Nutrition, 62(6), 1427S-1434S.
- Chowanadisai, , Bauerly, K.A., Tchaparian, E., Wong, A., Cortopassi, G.A., & Rucker, R.B. (2010). Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. The Journal of Biological Chemistry, 285(1), 142-152.
- Hwang, H., Kim, K.J., Ryu, S.J., & Lee, B.Y. (2012). Pyrroloquinoline quinone attenuates oxidative stress and inflammation in human endothelial cells. Journal of Medicinal Food, 15(8), 758-762.
- Harris, C.B., Chowanadisai, W., Mishchuk, D.O., Satre, M.A., Slupsky, C.M., & Rucker, R.B. (2013). Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. The Journal of Nutritional Biochemistry, 24(12), 2076-2084
- Stites, , Storms, D., Bauerly, K., Mah, J., Harris, C., Fascetti, A., & Rucker, R.B. (2006). Pyrroloquinoline quinone modulates mitochondrial quantity and function in mice. The Journal of Nutrition, 136(2), 390-396.
- Rucker, , Chowanadisai, W., & Nakano, M. (2009). Potential physiological importance of pyrroloquinoline quinone. Alternative Medicine Review, 14(3), 268-277.
- Crane, F.L. (2001). Biochemical functions of coenzyme Q10. Journal of the American College of Nutrition, 20(6), 591-598. DOI: 1080/07315724.2001.10719063.
- Bhagavan, H.N., & Chopra, R.K. (2006). Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radical Research, 40(5), 445-453. DOI: 10.1080/10715760600617843.
- Littarru, P., & Tiano, L. (2007). Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Molecular Biotechnology, 37(1), 31-37. DOI: 10.1007/s12033-007-0052-y.
- Beal, F. (1999). Coenzyme Q10 as a possible treatment for neurodegenerative diseases. Free Radical Biology and Medicine, 28(7-8), 1554- 1560. DOI: 10.1016/s0891-5849(99)00025-1.
- Di Lorenzo, , Iannuzzo, G., Parlato, A., Cuomo, G., Testa, G., Coppola, A., … & Calabrese, C. (2013). A randomized, double-blind, placebo- controlled study to evaluate the efficacy and tolerability of coenzyme Q10 in early Parkinson disease. Archives of Neurology, 61(6), 889- 892. DOI: 10.1001/archneur.61.6.889.
- Gibson, G. E., Hirsch, J. A., & Fonzetti, P. (2012). Vitamin B1 (thiamine) and dementia. Annals of the New York Academy of Sciences, 1259(1), 170-175. DOI: 10.1111/j.1749-6632.2012.06588.x
- Bettendorff, L., & Wins, P. (2009). Thiamine triphosphatase and the CYTH superfamily of proteins. The FEBS Journal, 276(3), 745-756. DOI: 10.1111/j.1742-4658.2008.06833.x.
- Lu’o’ng, V., & Nguyen, L. T. (2011). The role of thiamine in cancer: possible genetic and cellular signaling mechanisms. Cancer Genetics and Cytogenetics, 203(1), 1-7. DOI: 10.1016/j.cancergencyto.2010.09.010.
- Powers, H. J. (2003). Riboflavin (vitamin B-2) and health. The American Journal of Clinical Nutrition, 77(6), 1352-1360. DOI: 1093/ajcn/77.6.1352.
- Said, H. M. (2011). Riboflavin transport and metabolism in humans. Journal of Clinical Nutrition, 1(3), 115-124. DOI: 1016/j.clnu.2011.05.001.
- Vecino, R., Burguete, M. C., Jover-Mengual, T., & Agulla, J. (2017). Role of thiamine and thiamine metabolism in glutamate-induced Neurochemistry International, 105, 41-48. DOI: 10.1016/j.neuint.2017.01.016.
- Saedisomeolia, A., & Ashoori, M. (2014). Riboflavin in human health: A review of current evidence. Advances in Food and Nutrition Research, 71, 1-39. DOI: 10.1016/B978-0-12-800268-1.00001-9.
- Moeller, M., & Jacques, P. F. (2003). The potential role of dietary xanthophylls in cataract and age-related macular degeneration. Journal of the American College of Nutrition, 22(5), 242-256. DOI: 10.1080/07315724.2003.10719301.
- Gropper, S., & Smith, J. L. (2013). Advanced Nutrition and Human Metabolism (6th ed.). Wadsworth Cengage Learning.
- Higdon, J., Drake, V. J., & Delage, B. (2009). Pantothenic Acid. In L. J. M. Weaver et al. (Eds.), Micronutrient Information Center. Linus Pauling Institute, Oregon State University.
- Goswami, , Gupta, A., Sharma, S. K., & Sharma, A. (2014). Interplay between oxidative stress and cytokines in inflammatory bowel disease: A review. International Journal of Molecular and Cellular Medicine, 3(4), 205-215.
- Kaltschmidt, B., Kaltschmidt, C., & Hehner, S. P. (1999). NF-kappaB in the nervous system. Cold Spring Harbor Perspectives in Biology, 1(3), a001271
- Sanvictores, T., & Chauhan, S. (2003). Vitamin B5 (Pantothenic Acid). Clinical Guide to Nutrition & Dietary Supplements in Disease Management, 5, 699–701. https://doi.org/10.1016/B978-0-443-07193-5.50106-0
- Réus, Z., Fries, G. R., Stertz, L., Badawy, M., Passos, I. C., Barichello, T., Kapczinski, F., & Quevedo, J. (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience, 300, 141–154. https://doi.org/10.1016/j.neuroscience.2015.05.018
- Adrian, H., Mårten, K., & Salla, N. (2016). Biomarkers of Traumatic Brain Injury : Temporal Changes in Body Fluids. ENeuro, 000(December), 1–13. https://www.eneuro.org/content/eneuro/3/6/ENEURO.0294-16.2016.full.pdf
- Murugan, S., Jakka, P., Namani, S., Mujumdar, V., & Radhakrishnan, G. (2019). The neurosteroid pregnenolone promotes degradation of key proteins in the innate immune signaling to suppress inflammation. Journal of Biological Chemistry, 294(12), 4596–4607. https://doi.org/10.1074/jbc.RA118.005543
- Fitch, M. T., Doller, C., Combs, C. K., Landreth, G. E., & Silver, J. (1999). Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 19(19), 8182–8198. https://doi.org/10.1523/jneurosci.2488-08.2008
- Singh, , Kukreti, R., Saso, L., & Kukreti, S. (2019). Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules.
Molecules, 24(8), 1–20. https://www.nature.com/articles/npp2016185
- Bhat, H., Dar, K. B., Anees, S., Zargar, M. A., Masood, A., Sofi, M. A., & Ganie, S. A. (2015). Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomedicine and Pharmacotherapy, 74, 101–110. https://doi.org/10.1016/j.biopha.2015.07.025
- Gordon, M. L. (2023). Neuroinflammation as a Barrier to the Success of Psychedelic-assisted Therapies. (Issue 8). https://www.tbihelpnow.org/the-science

