S-ADENOSYLMETHIONINE (SAMe) – AN EXCITING NEW ANTI-AGING THERAPY
Part 1: This is the first in a series of articles on S-Adenosylmethionine (SAMe), which promises to be the most potent, multi-purpose anti-aging, anti-disease therapy we have ever introduced! A natural metabolite of the amino acid methionine, SAMe appears to be a non-toxic, anti-aging, health-enhancing blockbuster that could do wonders for every one of us.
https://www.lifeextension.com/magazine/1997/3/report
Ronald Peters, MD- Comments
In proper balance, SAMe is highly benefcial to your body and mind. However, more is not better. High doses can disrupt the complex chemistry of the body and potentially cause side-effects. I suggest sticking to 200mg twice daily on an empty stomach.
What Does SAMe Do?
It’s hard to believe the many reports in the scientific literature about the potential health and medical applications of SAMe. It was only after we read about SAMe’s mechanisms of action within the body that we began to understand how one substance could have so many beneficial effects against aging and disease.
On a cellular level, SAMe:
- Maintains mitochondrial function
- Prevents DNA mutations
- Restores cellular membrane fluidity so that cell receptors become better able to bind hormones and other factors
In addition, SAMe:
- Protects the liver against alcohol, drugs and cytokines. It protects against cholestasis (bile impairment or blockage). It may protect against chronic active hepatitis. It protects against liver damage caused by MAO inhibitors and anti-convulsants. It reverses hyperbilirubinaemia.
- Protects against neuronal death caused by lack of oxygen (anoxia). It regenerates nerves and provokes remyelination of nerve fibers.
- May protect against heart disease
Moreover, SAMe has antidepressant action equal to, and faster than FDA-approved drugs, and is essential for the synthesis of melatonin.
What is SAMe?
SAMe, (which is also known as SAM or AdoMet) is a synthetic form of a natural metabolite of the amino acid methionine. SAMe is of great importance because it is a co-factor in a number of critical biochemical reactions. SAMe is the precursor for three fundamental biochemical pathways and it is found in almost every tissue of the body. SAMe has been used in clinical studies to treat depression, schizophrenia, demyelination diseases, liver disease, dementia, arthritis, and other conditions. It was recently published in the Journal of Neurochemistry that brain levels of SAMe in Alzheimer’s patients are severely decreased.
Figure 1 Melationin Sythesis in the Pineal and its Relationship with SAMe
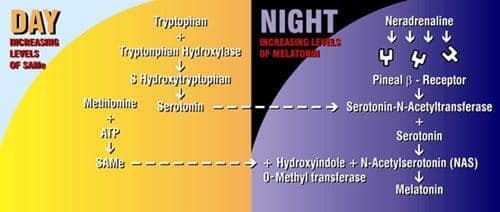
SAMe and serotonin produced during the day facilitate melatonin production during the night. Blocking of B receptors by beta-blocking drugs disrupts synthesis of melatonin. Serotonin is also produced at night.
SAMe Is Necessary for Melatonin
One of the most exciting things about SAMe is that it is melatonin’s daytime equivalent. The natural synthesis of melatonin during the night is dependent on the synthesis of SAMe during the day. SAMe is necessary for the biochemical reaction that converts serotonin into melatonin. (Serotonin is the neurotransmitter that drugs like Prozac elevate). SAMe and melatonin are entwined in a circadian rhythm that see-saws back and forth as the sun rises and sets. SAMe is melatonin’s other half: when melatonin levels shoot up at night, SAMe stays low. But during the day, when melatonin falls, SAMe levels climb. Without adequate SAMe during the day, neither melatonin nor serotonin can be synthesized. And both are dependent on light and dark.
One of the most fascinating animal studies on SAMe and melatonin was published in the Journal of Neurochemistry in 1995. Researchers demonstrated in great detail the perfect orchestration that occurs between levels of SAMe and melatonin. The so-called “nyctohemeral” rhythm (pertaining to both day and night) was documented almost minute-by-minute. Data were translated onto graphs showing the see-saw relationship between melatonin and SAMe (Fig. 1).
Both melatonin and SAMe are controlled by an internal “clock” that knows lightness from darkness. In the evening, about 30 minutes before sunset, levels of SAMe shoot up to their highest level. They stay there for about an hour, and then suddenly drop. When this happens, melatonin kicks in. Melatonin increases for four hours, while SAMe drops. Five hours into the night, melatonin hits its high, and SAMe hits its low. Melatonin stays elevated until three hours before sunrise, when it abruptly falls. Meanwhile, SAMe builds up. Five hours into the day (around 11:00 A.M.), SAMe reaches its peak level again, then begins a gradual descent until evening.
Serotonin levels follow roughly the same pattern-higher during the day and lower at night. It appears that the serotonin synthesized during the day is used at night to make melatonin. SAMe is absolutely crucial for the natural synthesis of melatonin because it donates a methyl group molecule to the enzyme that converts the acetylated form of serotonin to melatonin.
Risks Of Beta Blockers
The synthesis of melatonin also depends on the stimulation of pineal beta receptors by noradrenaline (norepinephrine). This normally occurs as the sun goes down. Noradrenaline stimulation of beta receptors causes the release of Serotonin-N-Acetyltransferase, an enzyme crucial for the synthesis of melatonin.
Beta blockers are a class of drugs used to treat heart problems. Common brand names include Inderal, Tenormin, Lopressor (Toprol). People who take beta blockers to block beta receptors in their heart, block receptors in their brains as well. Blocking neal receptors interferes with the synthesis of melatonin.
The extent of the disruption caused by beta blockers to circadian rhythms, and the important hormones that depend on these rhythms, was illustrated by an animal study showing that ingesting a beta blocker before bedtime is like leaving the lights on all night. Serotonin levels, which normally drop at night, remain unnaturally high: SAMe doesn’t decrease, and melatonin doesn’t increase. What occurs when beta blockers are taken before bedtime looks a lot like sleep deprivation.
A study in Acta Medica Scandinavia clearly demonstrates the melatonin-disrupting effects of beta blockers. Researchers gave hypertensive patients one of three beta-blockers: propranolol (Inderal), atenolol (Tenormin), or metoprolol (Lopressor or Toprol XL). Melatonin levels were measured against those of a control group. As a whole, melatonin levels fell significantly in patients compared to controls, and wakefulness increased. Metoprolol decreased melatonin more than atenolol or propranolol. The three patients with the lowest levels of melatonin reported nightmares. People do not “adjust” to the chronic use of beta-blockers: as long as they take them, melatonin will be suppressed.
People who take beta blockers (at least in the evening) are setting themselves up for insomnia, depression and other mental disturbances due to the extraordinary interdependence of SAMe, serotonin and melatonin. Beta blockers suppress serotonin as well as melatonin. Thus, it is not surprising that the Physicians Desk Reference lists mental depression, fatigue, short-term memory loss, insomnia and emotional lability as some of the side effects of Inderal and other beta blockers. Apparently unaware of the role beta receptors play in getting a good night’s sleep, the manufacturer of Inderal, Wyeth-Ayerst, recommends that these drugs be taken at bedtime.
Anti-Anxiety Pills Suppress Melatonin
Two benzodiazepine type drugs have been shown to suppress melatonin and disrupt sleep patterns in humans and rodents. Valium and Xanax both cause decreased production of melatonin at night. In a study from the Niigata College of Pharmacy in Japan, Valium also inhibited N-acetylserotonin and N-acetyltransferase, the enzymes necessary for the synthesis of melatonin in the pineal gland.
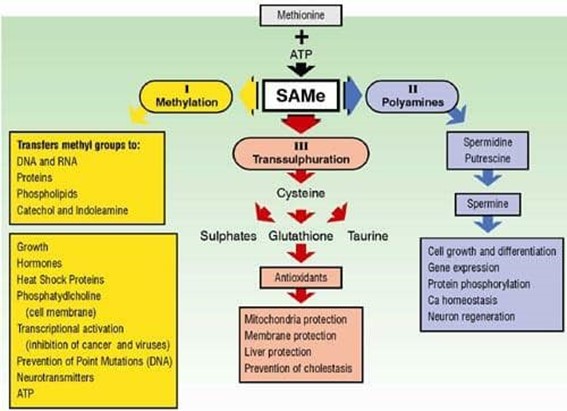
The Three major pathways of SAMe
SAMe Against Killer Diseases
A recent study in the Journal of Neurochemistry reported that Alzheimer’s disease (AD) patients have severely decreased levels of SAMe in their brains. This is an important discovery because it was previously assumed from studies on blood cells that AD patients had too much SAMe. Proper studies have never been done with SAMe as a treatment for Alzheimer’s disease because of this erroneous assumption.
A new study in Arteriosclerosis Thrombosis and Vascular Biology links coronary artery disease to SAMe. This well-constructed study in Switzerland measured levels of SAMe, cholesterol and other factors in 70 patients, aged 28-79, who were admitted to the hospital for angioplasty. The researchers became suspicious that SAMe could be playing a role in heart disease when they read studies revealing high levels of homocysteine in heart patients. Homocysteine can be neutralized by a process that involves SAMe, which has led researchers at Tufts University to propose that a disruption in one SAMe pathway could affect other pathways, resulting in homocysteine accumulation.
The Swiss researchers showed for the first time that high levels of homocysteine in heart disease correlate with an enzyme (5-methyltetrahydrofolate) that converts folate into its active form. SAMe plays a crucial role in keeping this enzyme from breaking down. It also participates in other processes which turn homocysteine back into methionine. There was clear correlation between high homocysteine, low SAMe and heart disease.
To make sure that heart disease causes the high levels of homocysteine, rather than the other way around, the researchers measured homocysteine levels again after a year. They concluded that “The finding of similar homocysteine values in patients after an interval of 1 year supports the idea that this parameter plays a role in the disease process and is not just altered by the disease itself.” A study in JAMA in 1992 supports the view that high levels of homocysteine precede coronary artery disease.
Cholesterol and Heart Disease
The study had another, unintentional, finding: some of the healthy controls had higher levels of cholesterol than the patients with heart disease. A recent review in Archives of Internal Medicine dealt with 24,968 people in 34 studies who participated in trials of cholesterol-lowering regimens to prevent coronary artery disease. The reviewers found that while lowering cholesterol by 10% or more reduced the risk of dying of a heart attack in middle-aged males somewhat, no conclusions could be reached for women and elderly people.
It appears that cholesterol may have less to do with heart disease than the public has been led to believe. Durk Pearson and Sandy Shaw questioned the causal connection between cholesterol and heart disease 14 years ago in their book, Life Extension. The recent data on homocysteine and heart disease suggests that levels of SAMe and its opposite, homocysteine, have more to do with heart disease than cholesterol.
A similar review looked at the association between the symptoms of peripheral atherosclerosis and cholesterol levels in people who have had heart attacks. The reviewers found that there was no association between cholesterol levels below 240 md/dl and heart attacks.
did, however, find that smoking, age, diabetes, previous heart attacks, hypertension, high blood pressure, and low alcohol consumption were risk factors for heart attacks. There is more persuasive evidence for the association between vitamin E and heart disease than there is for high cholesterol and heart disease.
Due to its function in every cell in the body, the potential of SAMe to reverse disease appears to be limitless. A study in Hepatology showed the beneficial effects of SAMe on liver disease. Fetal rat liver cells were exposed to ethanol. Ethanol caused a 40% reduction in DNA synthesis, a doubling of free radicals, a 30% decrease in cellular ATP, altered mitochondrial function, and cell damage. Pretreatment with SAMe maintained cell replication, decreased free radicals and prevented ATP and glutathione depletion. (Vitamin E gave better protection against free radicals, but did not maintain cell replication, ATP or glutathione). This one study illustrates all three important actions of SAMe.
Resources:
Morrison LD, Smith DD and SJ Kish. 1996. Brain S-adenosylmethionine levels are severely decreased in Alzheimer’s disease. J Neurochem 67: 1328-1331.
Loehrer MTF, Angst CP, Haefeli WE, Jordan PP, Ritz R and B Fowler. 1996. Low whole-blood S-adenosylmethionine and correlation between 5- methyltetrahydrofolate and homocysteine in coronary artery disease. Arth Throm Vasc Biol 16: 727-733.
McIntyre IM, Norman TR, Burrows GD, Armstrong SM. 1993. Alternations to plasma melatonin and cortisol after evening alprazolam administration in humans. Chronobiol Int 10: 205-13.
Wakabayashi H, Shimada K and T Satoh. 1991. Effects of diazepam administration on melatonin synthesis in the rat pineal in vivo. 1991. Chem Pharm Bull 39: 2674-6.
Sitaram BR, Sitaram M, Traut M and CB Chapman. 1995. Neurochem 65: 1887-1894.
Brismar K, Hylander B, Elilasson K, Rossner S and Wetterberg L. 1988. Melatonin secretion related to side-effects of beta-blockers from the central nervous system. Acta Med Scand 223: 525-30.
Marcholi R, Marfisi RM, Carinci F and G Tognoni. 1996. Meta-analysis, clinical trials, and transferability of research results into practice. The case of cholesterol- lowering intervention in the secondary prevention of coronary heart disease. Arch Intern Med 156: 1158-72.
Stampfer MJ, Malinow MR, Willett WC, et al. 1992. A prospective study of plasma homocysteine and risk of myocardial infarction in US physicians. JAMA 268: 877-81.

